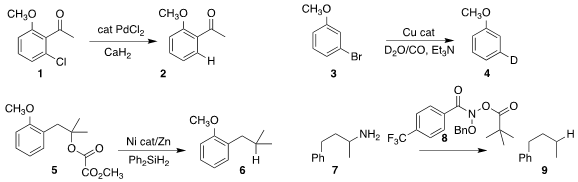
Xiaobei Chen,Yanwei Hu and Shilei Zhang of Soochow University showed that the
inexpensive calcium hydride could be used to
reduce the aryl halide 1 to the arene
2
(Org. Chem. Front. Formula of 131726-65-3 2021, 8, 4685.
DOI: 10.1039/D1QO00758K).
Kathrin Junge and Matthias Beller of
the Leibniz-Institut für Katalyse showed that the water-gas shift reaction could
be used to reduce the aryl bromide 3, leading to the deuterated arene 4
(Chem. Sci. 2021, 12, 14033.
DOI: 10.1039/D1SC04259A).
Hegui Gong of Shanghai University used a Ni catalyst to prepare 6 by the
deoxygenation of the oxalate 5
(Synlett 2021, 31, 1625.
DOI: 10.1055/a-1328-0352).
Osvaldo Gutierrez, now at Texas A&M University, and
Mark D. (Bromomethyl)cycloheptane structure Levin of the University of Chicago used the reagent 8 to prepare 9 by the
reductive
deamination of 7
(J. PMID:23892746 Am. Chem. Soc. 2021, 143, 17366.
DOI: 10.1021/jacs.1c09779).
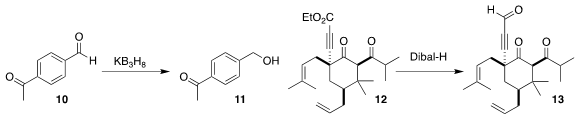
Yan-Na Ma and Xuenian Chen of Zhengzhuo University demonstrated the
convenience of KB3H8 as a reagent for organic synthesis, selectively reducing
the keto aldehyde 10 to the keto alcohol 11
(Chem. Commun. 2021, 57, 12776.
DOI: 10.1039/D1CC05638G).
Chulbom Lee of Seoul National University demonstrated that the alkynoate of 12 could be
selectively reduced to the aldehyde
13 in the presence of the two
ketones
(Angew. Chem. Int. Ed. 2021, 60, 22735.
DOI: 10.1002/anie.202109193).
Ya Kawamata and Phil S. Baran
of Scripps-La Jolla used alternating current to reduce the imide of 14 to the
lactam 15, leaving the aldehyde unchanged
(J. Am. Chem. Soc. 2021, 143, 16580.
DOI: 10.1021/jacs.1c06572).
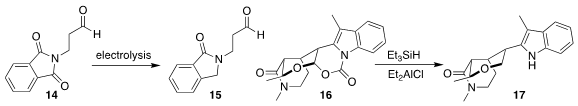
Stephen F. Martin of the University of Texas reduced and deprotected 16, leading
to the tetrahydropyran
17
(Tetrahedron 2021, 89, 132150.
DOI: 10.1016/j.tet.2021.132150).
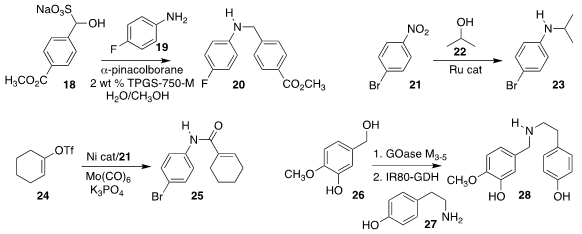
J. Daniel Bailey of Takeda Pharmaceuticals and Bruce H. Lipshutz of the
University of California, Santa Barbara demonstrated that using a surfactant,
the water-soluble aldehyde bisulfite addition product 18 could be used directly
in reductive amination with
19, leading to the amine 20
(Org. Lett. 2021, 23, 7205.
DOI: 10.1021/acs.orglett.1c02604).
Bao-Hua Xu of the Institute of Process Chemistry, Chinese Academy of
Sciences showed that using a Ru catalyst, the nitroaromatic 21 could be coupled
with the alcohol 22, leading to the
amine 23
(Org. Chem. Front. 2021, 8, 6710.
DOI: 10.1039/D1QO01269J).
Xinxin Qi of Zhejiang Sci-Tech University and Xiao-Feng Wu, also of the
Leibniz-Institut für Katalyse, coupled the enol triflate 24 with the
nitroaromatic 21, leading to the amide
25
(Org. Chem. Front. 2021, 8, 6974.
DOI: 10.1039/D1QO01508G).
Sebastian C. Cosgrove of the University of Keele and the University of Manchester and Sabine L. Flitsch of the University of Manchester
devised a flow system for the sequential application of otherwise incompatible
enzyme systems, allowing the net coupling of the alcohol 26 with the amine
27 to give 28
(Angew. Chem. Int. Ed. 2021, 60, 18660.
DOI: 10.1002/anie.202103805).
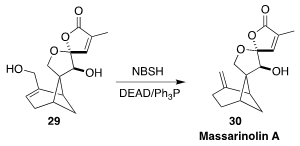
Massarinolin A (30), isolated from the aquatic fungus Massarina tunicata,
showed activity against gram-positive bacteria. For the last step in their total
synthesis, Mingji Dai of Purdue University used the Myers protocol to reduce the
allylic alcohol 29 to 30
(Angew. Chem. Int. Ed. 2021, 60, 24828.
DOI: 10.1002/anie.202109625).