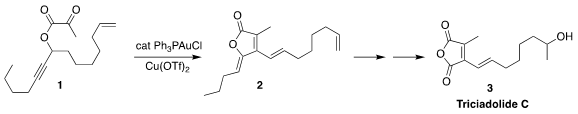
The maleic anhydride derivative triciadolide C (3) was isolated from
the aquatic hyphomycete Tricladium castaneicola. PMID:23460641 Shaozhong Wang of
Nanjing University developed a general route to such anhydrides, exemplified by
the gold-catalyzed cyclization of the propargyl pyruvate 1 to the lactone 2
(J. Org. Chem. 121553-38-6 site 2021, 86, 15318.
DOI: 10.1021/acs.joc.1c01890). 4-(Methylsulfinyl)aniline supplier
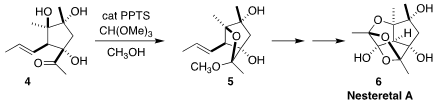
Nesteretal A (6) was isolated from the coral-derived actinomycete
Nesterenkonia habia. Hisanaka Ito of the Tokyo University of Pharmacy and
Life Sciences set the absolute configuration of the triol 4 by
Sharpless
asymmetric dihydroxylation of an acyclic precursor. Acid-mediated intramolecular
ketalization of 4 led to 5, that was carried on to 6
(Org. Lett. 2021, 23, 7074.
DOI: 10.1021/acs.orglett.1c02478).
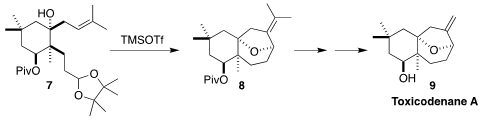
Toxicodenane (9) was isolated from the Chinese lacquer tree
Toxicodendron vernicifluum, used in traditional Chinese medicine. En route
to 9, Fu-She Han of the Chinese Academy of Sciences, Changchun devised
the transacetalization/Prins
cyclization of the acetal 7 to the tricyclic ether 8
(Org. Lett. 2021, 23, 8570.
DOI: 10.1021/acs.orglett.1c03293).
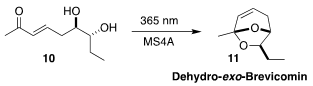
Dehydro-exo-brevicomin (11) is a pheromone of the mouse, Mus
musculus. Nobuhiro Kanomata of Waseda University also used Sharpless
asymmetric dihydroxylation to set the absolute configuration of the diol 10,
that on irradiation cyclized to 11
(Org. Biomol. Chem. 2021, 19, 6897.
DOI: 10.1039/D1OB00952D).
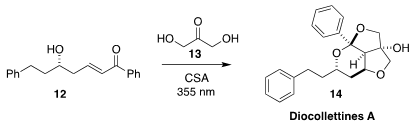
Diocollettines A (14), isolated from the Chinese perennial vine
Dioscorea collettii, showed modest anti-cancer activity. K. N. Houk of UCLA
and Amos B. Smith III of the University of Pennsylvania also used irradiation to
promote the coupling of the enone 12 with dihydroxyacetone 13,
leading to 14
(J. Org. Chem. 2021, 86, 13583.
DOI: 10.1021/acs.joc.1c01635).
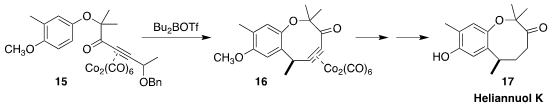
Heliannuol K (17) was isolated from the sunflower, Helianthus
annuus. James R. Green of the University of Windsor showed that the Nicholas
cyclization of 15 proceeded smoothly, to give the benzo[b]oxocane 16
(Org. Biomol. Chem. 2021, 19, 7152.
DOI: 10.1039/D1OB01395E).
We note the passing of Tomá Hudlický, who in addition to his many other
conributions made practical the enzymatic oxidation of benzene derivatives to
enantiomerically-pure carbocyclic starting materials.