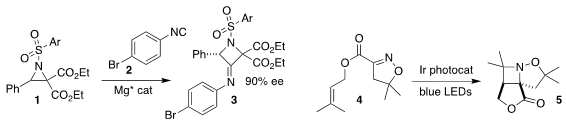
Weidi Cao and Xiaoming Feng of Sichuan University achieved high
enantioselectivity in the ring expansion of the racemic aziridine with the
isonitrile 2 to give the
azetidine 3
(Org. Lett. 2022, 24, 1513.
DOI: 10.1021/acs.orglett.2c00190).
Frances H. Arnold of Caltech also effected the enantioselective ring expansion
(not illustrated) of an aziridine to an azetidine
(J. Am. PMID:24360118 Chem. Soc. 2022, 144, 4739.
DOI: 10.1021/jacs.2c00251). Buy1438382-15-0
Corinna S. Schindler of the University of Michigan effected the
photochemical cyclization of the ester 4 to the azetidine 5
(Org. Lett. 2022, 24, 3053.
DOI: 10.1021/acs.orglett.2c01008).
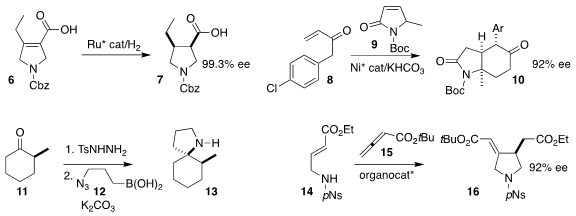
Michael J. Rozema of AbbVie achieved high ee in the hydrogenation of the acid 6
to the pyrrolidine 7
(Org. Process Res. Dev. 2022, 26, 949.
DOI: 10.1021/acs.oprd.1c00287).
Hongbo Wei and Weiqing Xie of Northwest A&F University constructed
the bicylic ketone 10 from the enone 8 and the lactam 9
(Org. Biomol. Chem. 2022, 20, 2387.
DOI: 10.1039/D2OB00112H). N-(3-Chloro-4-hydroxyphenyl)acetamide Order
Carlos Valdés of the University of Olviedo assembled the pyrrolidine 13 by combining the boronic
acid 12 with the diazo alkane prepared in situ from the tosylhydrazone of the ketone 11
(Angew. Chem. Int. Ed. 2022, 61, e202113370.
DOI: 10.1002/anie.202113370).
David W. Lupton of Monash University used an enantiomerically-pure phosphine to mediate the
addition of the sulfonamide 14 to the allene 15, leading to the pyrrolidine 16
(Org. Lett. 2022, 24, 2847.
DOI: 10.1021/acs.orglett.2c00785).
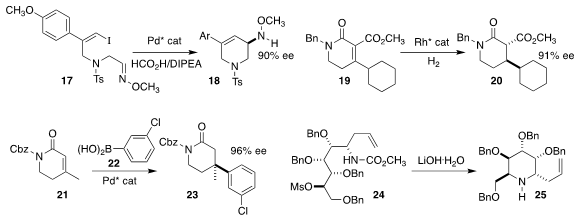
Xiaofeng Tong of Changzhou University effected the enantioselective reductive
Heck cyclization of 17, leading to the
piperidine 18
(Org. Lett. 2022, 24, 2457.
DOI: 10.1021/acs.orglett.2c00823).
Xumu Zhang of the Southern University of Science and Technology and Qin
Yin of the Shenzhen Institute of Advanced Technology achieved high ee in the
hydrogenation of the ester 19 to the
lactam 20
(Org. Lett. 2022, 24, 675.
DOI: 10.1021/acs.orglett.1c04132).
Sukwon Hong of the Gwangju Institute of Science and Technology also observed
high ee in the preparation of the lactam 23 by the conjugate addition of the
boronic acid 22 to the unsaturated lactam 21
(ACS Catal. 2022, 12, 5559.
DOI: 10.1021/acscatal.2c00541).
Young Hoon Jung of Sungkyunkwong University cyclized the sugar-derived mesylate
24 to the piperidine 25
(Tetrahedron 2022, 116, 132809.
DOI: 10.1016/j.tet.2022.132809).
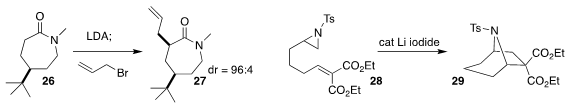
Keith A. Woerpel of New York University observed that alkylation of the
seven-membered ring lactam 26 led to 27 with high diastereocontrol
(Angew. Chem. Int. Ed. 2022, e202114183,
DOI: 10.1002/anie.202114183;
Org. Lett. 2022, 24, 6722,
DOI: 10.1021/acs.orglett.2c02445).
Siyang Xing, Kui Wang and Bolin Zhu of Tianjin Normal University effected intramolecular cycloaddition
of the aziridine 28 to the unsaturated ester, leading to the bicyclic sulfonamide 29
(J. Org. Chem. 2022, 87, 6426.
DOI: 10.1021/acs.joc.2c00287).
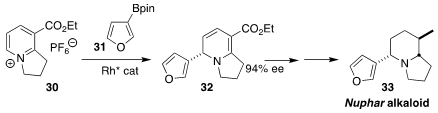
The Nuphar alkaloid (33) was isolated from the castoreum of the North American
beaver. Rashad R. Karimov of Auburn University established the absolute
configuration of 33 by the enantioselective addition of the boronate 31 to the pyridinium salt
30, leading to 32
(Org. Lett. 2022, 24, 3445.
DOI: 10.1021/acs.orglett.2c00976).