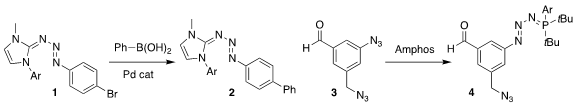
John C. Jewett of the University of Arizona demonstrated that the protected
diazonium salt 1 participated efficiently in Suzuki coupling, leading the
biphenyl 2
(Org. Lett. 2021, 23, 1851.
DOI: 10.1021/acs.orglett.1c00257).
Takamitsu Hosoya of the Tokyo
Medical and Dental University and Suguru Yoshida of the Tokyo University of
Science showed that the aryl azide of 3 could be protected in the presence of
the alkyl azide, to give 4
(Chem. PMID:23659187 Commun. 6-Bromo-5-fluoroisoquinolin-1(2H)-one In stock 2021, 57, 6062.
DOI: 10.1039/D1CC01143J). 2-Bromo-1-cyclohexylethan-1-one Formula
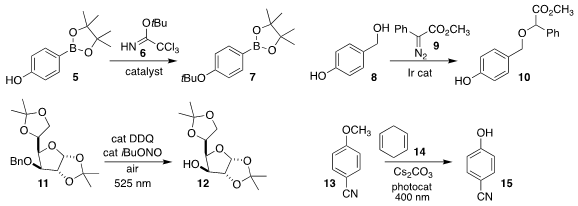
Keith R. Fandrick and Suttipol Radomkit of Boehringer-Ingelheim used the
reagent 6 and a bis(trifluoromethane)sulfonimide/2,6-lutidine catalyst to
convert the phenol 5 into the
t-butyl ether
7
(J. Org. Chem. 2021, 86, 4877.
DOI: 10.1021/acs.joc.1c00193).
Jie Zhao of the East China University of Science and Technology, Dingsheng Wang
of Tsinghua University and F. Dean Toste of the University of California,
Berkeley showed that with an Ir catalyst, the benzyl alcohol of 8 could be
coupled with the diazo ester 9 to give 10, leaving the phenol unreacted
(Nature Catal. 2021, 4, 523.
DOI: 10.1038/s41929-021-00637-7).
Bartholomäus Pieber of the Max Planck Institute of
Colloids and Interfaces and Peter H. Seeberger of that institution and the Freie
Universität Berlin devised oxidative conditions promoted by long-wavelength
visible light to prepare the free alcohol 12 from the
benzyl ether 11
(Org. Lett. 2021, 23, 514.
DOI: 10.1021/acs.orglett.0c04026).
Ryosuke Matsubara of Kobe University showed that 14 and a carbazole photocatalyst mediated the
cleavage of the aryl ether
13 to the phenol 15
(J. Org. Chem. 2021, 86, 2545.
DOI: 10.1021/acs.joc.0c02663).
The protocol cleaved long chain alkyl aryl ethers.
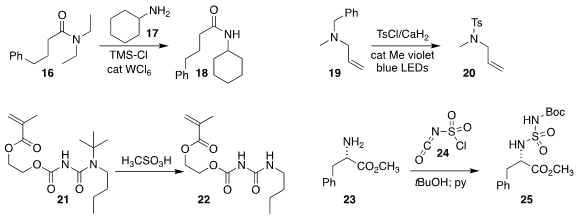
Chi Wai Cheung and Jun-An Ma of Tianjin University showed showed that a primary amine 17
could be acylated with a tertiary amide
16, leading to the secondary amide 18
(ACS Catal. 2021, 11, 7070.
DOI: 10.1021/acscatal.1c01840).
Ying Fu and Zhengyin Du of Northwest Normal University developed photochemical conditions for converting the
benzyl amine 19 to the sulfonamide 20
(Eur. J. Org. Chem. 2021, 1896.
DOI: 10.1002/ejoc.202100144).
Jianjun Cheng of
the University of Illinois removed the t-butyl group from the urea 21 to give
the urea 22
(Chem. Commun. 2021, 57, 3812.
DOI: 10.1039/D1CC00715G).
Chun Hu of Shenyang Pharmaceutical
University and Dong-Yu Wang of Shanghai Jiao Tong University combined
chlorosulfonyl isocyanate 24 with tBuOH, then pyridine, to form a reagent that
converted the amine 23 to the protected
sulfamide 25
(Org. Lett. 2021, 23, 2595.
DOI: 10.1021/acs.orglett.1c00504).
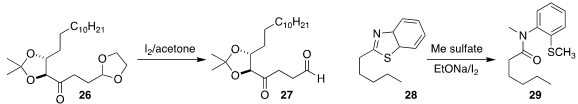
On deprotection, 26 tends to form the internal acetal. Shivajirao L. Gholap
of the Indian Institute of Technology Delhi developed conditions that delivered
the aldehyde 27
(Org. Biomol. Chem. 2021, 19, 1100.
DOI: 10.1039/D0OB02303E).
Fan Chen and Xing-Guo Zhang
of Wenzhou University showed that the
benzothiazole 28 could be converted into
the amide 29
(Chem. Commun. 2021, 57, 1923.
DOI: 10.1039/D0CC08096A).
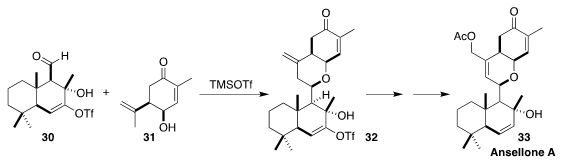
Ansellone A (33), isolated from the dorid nudibranch Cadlina luteomarginata,
has activity as an HIV latency-reversing agent. In the course of a synthesis of
33, Kenichi Murai and Mitsuhiro Arisawa of Osaka University demonstrated that
the alkenyl triflate 30 participated more efficiently in the coupling with
31 to give 32 than did the corresponding alkene
(Org. Lett. 2021, 23, 1720.
DOI: 10.1021/acs.orglett.1c00151).