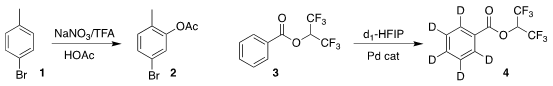
Duen-Ren Hou of the National Central University devised a simple protocol for the
direct acetoxylation of the substituted benzene 1, leading to the aryl acetate 2
(Org. Lett. 2021, 23, 8127.
DOI: 10.1021/acs.orglett.1c02183).
Manuel van Gemmeren of Westfälische Wilhelms-Universität Münster demonstrated the facile
conversion of the benzoate 3 to the ring-deuterated benzoate 4
(J. Am. Chem. Soc. 2021, 143, 16370.
DOI: 10.1021/jacs.1c08233). Buy2,4-Dimethyl-1H-pyrrole
Bill Morandi of ETH Zürich used 1-iodobutane (6) to convert the benzoic acid 5
to the iodobenzene 7
(Angew. PMID:32261617 Chem. Int. Ed. 2021, 60, 17211.
DOI: 10.1002/anie.202103269).
Weiran Yang of Nanchang University described a parallel investigation with anthranilic acids
(Org. Chem. Front. 2021, 8, 4479.
DOI: 10.1039/D1QO00461A).
Tobias Ritter of the Max-Planck-Institut für Kohlenforschung prepared the
phenol 9 directly from the lithium benzoate 8
(Angew. 957135-12-5 Formula Chem. Int. Ed. 2021, 60, 24012.
DOI: 10.1002/anie.202108971).
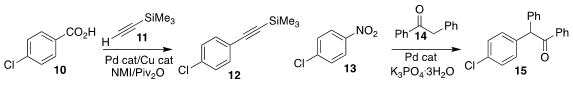
Ferdinand H. Lutter and Matthieu Jouffroy of Janssen Pharmaceutica assembled
the aryl alkyne 12 by coupling the benzoic acid
10 with TMS-acetylene 11
(Chem. Eur. J. 2021, 27, 14816.
DOI: 10.1002/chem.202102130).
Tao Wu, also of Nanchang University, prepared the
α-aryl ketone 15
by coupling the ketone 14 with the nitrobenzene derivative 13
(Org. Lett. 2021, 23, 881.
DOI: 10.1021/acs.orglett.0c04104).
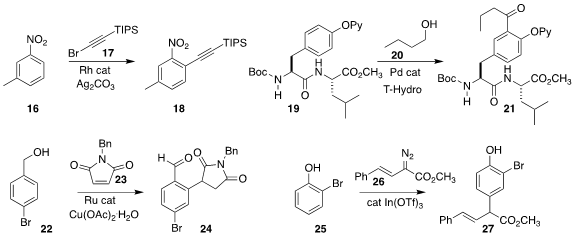
Antonio M. Echavarren of ICIQ used the bromoalkyne 17 to effect the ortho alkynylation of the nitroaromatic
16, leading to 18
(Chem. Sci. 2021, 12, 14731.
DOI: 10.1021/acs.orglett.1c02764).
Arkaitz Correa of the University of the Basque Country showed
that pendant tyrosines on even complex polypeptides could be ring-acylated with
primary alcohols, as exemplified by the preparation of 21 by the coupling of
19 with 1-butanol 20
(Org. Lett. 2021, 23, 7279.
DOI: 10.1021/acs.orglett.1c02764).
Dattatraya H. Dethe of the Indian
Institute of Technology Kanpur used a Ru catalyst to mediate the assembly of the
aryl succinimide 24 by the addition of the benzyl alcohol 22 to the
N-benzyl maleimide 23
(Org. Lett. 2021, 23, 6267.
DOI: 10.1021/acs.orglett.1c02040).
Yuanyuan Liu of East China Normal
University observed high preference for the para product 27 from the coupling of
the α-diazo ester 26 with the phenol 25
(Org. Chem. Front. 2021, 8, 6252.
DOI: 10.1039/D1QO01184G).
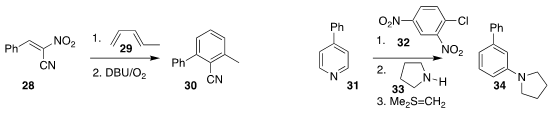
Nagatoshi Nishiwaki of the Kochi University of Technology devised a practical
preparation of nitroacetonitrile, allowing ready preparation of the substituted
acrylonitrile 28, that could be combined with the diene 29 to give, after
elimination and oxidation, the benzonitrile 30
(J. Org. Chem. 2021, 86, 13177.
DOI: 10.1021/acs.joc.1c01515).
Tatsuya Morofuji and Naokazu Kano of Gakushin University used
dinitrochlorobenzene 32 and pyrrolidine 33 to convert the pyridine
31 to the biphenyl 34
(Org. Lett. 2021, 23, 6126.
DOI: 10.1021/acs.orglett.1c02225).
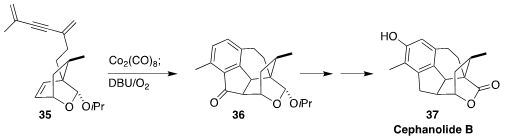
The cephanolide diterpenoids isolated from the coniferous shrub Cephalotaxus
sinensis, exemplified by cephanolide B (37), show antineoplastic, antiviral and
antitumor properties. Zhen Yang and Zichun Zhang of Peking University Shenzhen
Graduate School envisioned that
Pauson-Khand cyclization of the alkyne
35 would
be followed by 6π-electrocyclization to give an intermediate that could be
aromatized, leading to the ketone 36
(Org. Lett. 2021, 23, 9237.
DOI: 10.1021/acs.orglett.1c03579).