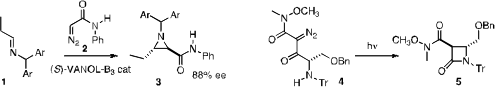
William D. Wulff of Michigan State University developed
(J. Am. Chem. 3-(Benzyloxy)cyclobutanone Chemscene Soc. 2010, 132, 13100,
DOI: 10.1021/ja1038648;
Org. Lett. 2010, 12, 4908,
DOI: 10.1021/ol102064b)
a general enantio- and diastereocontrolled route from an imine 1 to the
aziridine 3. Craig W. PMID:24605203 Lindsley of Vanderbilt University established
(Org. Lett. 2010, 12, 3276.
DOI: 10.1021/ol101276x)
a complementary approach, not illustrated. Joseph P. Konopelski of
the University of California, Santa Cruz designed
(J. Am. Chem. 758684-29-6 Formula Soc. 2010, 132, 11379.
DOI: 10.1021/ja1050023)
a practical and inexpensive flow apparatus for the cyclization of 4
to the β-lactam 5.
Manas K. Ghorai of the Indian Institute of Technology, Kanpur showed
(J. Org. Chem. 2010, 75, 6173.
DOI: 10.1021/jo101004x)
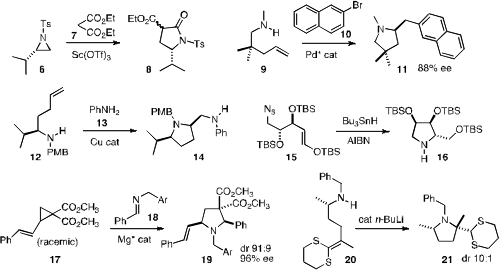
that an aziridine 6 could be opened with malonate to give the
γ-lactam 8. John P. Wolfe of the University of Michigan devised
(J. Am. Chem. Soc. 2010, 132, 12157.
DOI: 10.1021/ja106989h)
a Pd catalyst for the enantioselective cyclization of 9 to 11.
Sherry R. Chemler of the State University of New York at Buffalo observed
(Angew. Chem. Int. Ed. 2010, 49, 6365.
DOI: 10.1002/anie.201003499)
that the cyclization of 12 to 14 proceeded with high diastereoselectivity.
Glenn M. Sammis of the University of British Columbia devised
(Synlett 2010, 3035.
DOI: 10.1055/s-0030-1259062)
conditions for the radical cyclization of 15 to 16.
Jeffrey S. Johnson of the University of North Carolina observed
(J. Am. Chem. Soc. 2010, 132, 9688.
DOI: 10.1021/ja1032277)
that the opening of racemic 17 with 18 could be effected
with high ee. The residual 17 was highly enriched in the non-reactive enantiomer.
Kevin D. Moeller of Washington University found
(Org. Lett. 2010, 12, 5174.
DOI: 10.1021/ol102193x)
that the n-BuLi catalyzed cyclization of 20 set the quaternary center of
21 with high relative control.
Yujiro Hayashi of the Tokyo University of Science, using the diphenyl
prolinol TMS ether that he developed as an
organocatalyst, designed
(Org. Lett. 2010, 12, 4588.
DOI: 10.1021/ol1018932)
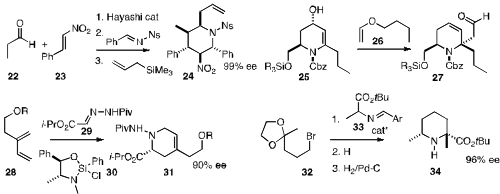
the sequential four-component coupling of 22, 23, benzaldehyde imine
and allyl silane to give 24 with high relative and absolute stereocontrol.
Derrick L. J. Clive of the University of Alberta showed
(J. Org. Chem. 2010, 75, 5223.
DOI: 10.1021/jo101010c)
that 25, prepared in enantiomerically-pure form from serine, participated
smoothly in the Claisen rearrangement, to deliver 27.
James L. Leighton of Columbia University extended
(J. Am. Chem. Soc. 2010, 132, 10248.
DOI: 10.1021/ja104480g)
the utility of the inexpensive chiral auxiliary 30, using it to effect the combination
of 28 and 29 to give 31. Keiji Maruoka of Kyoto University observed
(Chem. Sci. 2010, 1, 499.
DOI: 10.1039/C0SC00250J)
that the enamine from the alkylation of 33 with 32 could be hydrogenated
with high diastereocontrol to give 34.
Armen Zakarian of the University of California, Santa Barbara was able
(Org. Lett. 2010, 12, 4224.
DOI: 10.1021/ol101523z)
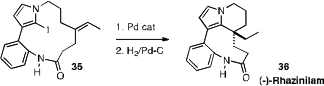
to separate the two enantiomers of 35 by HPLC on a chiral
stationary phase. Transannular Heck cyclization followed by hydrogenation then
converted 35 to (-)-Rhazinilam (36).