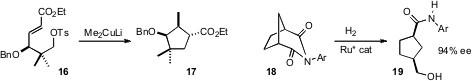Varinder K. Aggarwal of the University of Bristol described
(Angew. Chem. Int. Ed. 2010, 49, 6673.
DOI: 10.1002/anie.201003236)
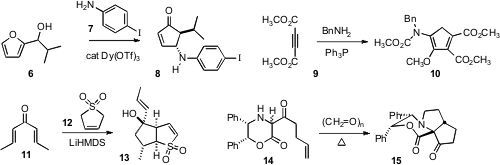
the conversion of the Sharpless-derived epoxide 1 into the
cyclopropane 2. Buy6-Fluoro-4-iodopyridin-3-ol Christopher D. Bray of Queen Mary University of London established
(Chem. Commun. 2010, 46, 5867.
DOI: 10.1039/C0CC01333A)
that the related conversion of 3 to 5 proceeded with high diastereocontrol.
Javier Read de Alaniz of the University of California, Santa Barbara extended
(Angew. Chem. Int. Ed. 2010, 49, 9484.
DOI: 10.1002/anie.201005131)
the Piancatelli rearrangement of a furyl carbinol 6 to allow inclusion of an amine
7, to give 8. Issa Yavari of Tarbiat Modares University described
(Synlett 2010, 2293.
DOI: 10.1055/s-0030-1258027)
the dimerization of 9 with an amine to give 10.
Jeremy E. Wulff of the University of Victoria condensed
(J. Org. PMID:23672196 Chem. 2010, 75, 6312.
DOI: 10.1021/jo101389c)
the dienone 11 with the commercial butadiene sulfone 12 to give the highly
substituted cyclopentane 13. Bromo-PEG1-CH2-Boc In stock Robert M. Williams of Colorado State University showed
(Tetrahedron Lett. 2010, 51, 6557.
DOI: 10.1016/j.tetlet.2010.10.037)
that the condensation of 14 with formaldehyde delivered the
cyclopentanone 15 with
high diastereocontrol. D. Srinivasa Reddy of Advinus Therapeutics Pvt. Ltd. devised
(Tetrahedron Lett. 2010, 51, 5291.
DOI: 10.1016/j.tetlet.2010.07.166)
conditions for the tandem conjugate addition/intramolecular alkylation conversion
of 16 to 17. Marie E. Krafft of Florida State University reported
(Synlett 2010, 2583.
DOI: 10.1055/s-0030-1258777)
a related intramolecular alkylation protocol.
Takao Ikariya of the Tokyo Institute of Technology effected
(J. Am. Chem. Soc. 2010, 132, 11414.
DOI: 10.1021/ja105048c)
the enantioselective Ru-mediated hydrogenation of bicyclic
imides such as 18. This transformation worked equally well for three-, four-,
five-, six- and seven-membered rings.
Stefan France of the Georgia Institute of Technology developed
(Org. Lett. 2010, 12, 5684.
DOI: 10.1021/ol102497w)
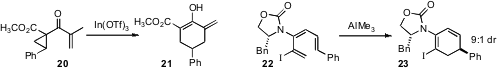
a catalytic protocol for the homo-Nazarov rearrangement of the doubly-activated cyclopropane
20 to the cyclohexanone 21. Richard P. Hsung of the University of Wisconsin effected
(Org. Lett. 2010, 12, 5768.
DOI: 10.1021/ol102693e)
the highly diastereoselective rearrangement of the triene 22 to the cyclohexadiene 23.
Strategies for polycyclic construction are also important. Sylvain Canesi of the Université de Québec devised
(Org. Lett. 2010, 12, 4368.
DOI: 10.1021/ol101851z)
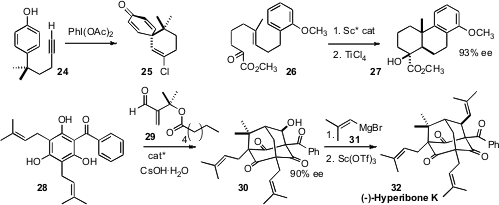
the oxidative cyclization of 24 to 25. Teck-Peng Loh of Nanyang Technological University demonstrated
(J. Am. Chem. Soc. 2010, 132, 10242.
DOI: 10.1021/ja104119j)
the product from enantioselective intramolecular ene cyclization of the β-keto ester 26 could be
further cyclized on exposure to TiCl4 to give the valuable trans-fused angularly methylated tricycle
27.
John A. Porco, Jr. of Boston University envisioned
(J. Am. Chem. Soc. 2010, 132, 13642.
DOI: 10.1021/ja1057828)
the spectacular condensation of 29 with 28 to give 30. Exposure of 30 to 31
followed by treatment with Lewis acid completed a concise synthesis of (-)-Hyperibone K (32).