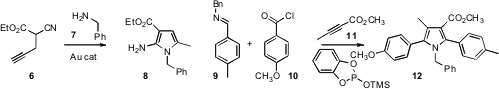
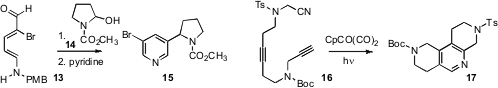
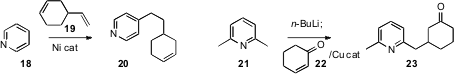
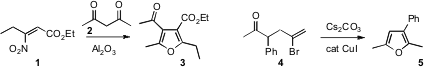Alessandro Palmieri of the University of Camerino developed
(Synlett 2010, 2468.
DOI: 10.1055/s-0030-1258031)
the condensation of a nitro acrylate 1 with a 1,3-dicarbonyl partner 2
to give the furan 3. Chaozhong Li of the Shanghai Institute of Organic Chemistry showed
(Tetrahedron Lett. 2010, 51, 3678.
DOI: 10.1016/j.tetlet.2010.05.040)
that an alkenyl halide 4 could be cyclized to the furan 5.
Ayhan S. Demir of Middle East Technical University established
(Chem. Commun. PMID:34645436 2010, 46, 8032.
DOI: 10.1039/C0CC02357D)
that a gold catalyst could catalyze the addition of an amine 7 to a cyanoester
6 to give the pyrrole 8. Bruce A. Price of 2′,3′-Dideoxy-5-iodouridine Arndtsen of McGill University effected
(Org. Lett. 2010, 12, 4916.
DOI: 10.1021/ol102075y)
the net three component coupling of an imine 9,
an acid chloride 10, and an alkyne 11 to deliver the pyrrole 12.
Bernard Delpech of CNRS Gif-sur-Yvette prepared
(Org. Price of 7361-31-1 Lett. 2010, 12, 4760.
DOI: 10.1021/ol101783c)
the pyridine 15 by combining the diene 13 with the incipient carbocation
14. Max Malacria, Vincent Gandon and Corinne Aubert of UPMC Paris optimized
(Synlett 2010, 2314.
DOI: 10.1055/s-0030-1258041)
the internal Co-mediated cyclization of a nitrile alkyne 16
to the tetrasubstituted pyridine 17. Yoshiaki Nakao of Kyoto
University and Tamejiro Hiyama, now at Chuo University, effected
(J. Am. Chem. Soc. 2010, 132, 13666.
DOI: 10.1021/ja106514b)
selective substitution of a preformed pyridine 18 at the C-4
position by coupling with an alkene 19. We
(J. Org. Chem. 2010, 75, 5737.
DOI: 10.1021/jo100890s)
showed that the anion from deprotonation of a pyridine 21
could be added in a conjugate sense to 22 to give 23. Other
particularly useful strategies for further substitution of preformed pyridines
have been described by Olafs Daugulis of the University of Houston
(Org. Lett. 2010, 12, 4277.
DOI: 10.1021/ol101684u),
by Phil S. Baran of Scripps/LaJolla
(J. Am. Chem. Soc. 2010, 132, 13194.
DOI: 10.1021/ja1066459),
and by Robert G. Bergmann of the University of California,
Berkeley and Jonathan A. Ellman of Yale University
(J. Org. Chem. 2010, 75, 7863.
DOI: 10.1021/jo101793r).
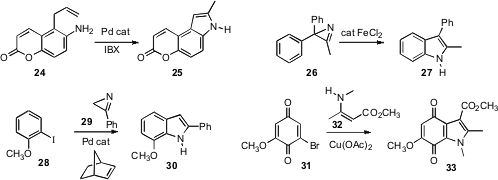
K. C. Majumdar of the University of Kalyani developed
(Tetrahedron Lett. 2010, 51, 3807.
DOI: 10.1016/j.tetlet.2010.05.068)
the oxidative Pd-catalyzed cylization of 24 to the
indole
25. Nan Zheng of the University of Arkansas showed
(Org. Lett. 2010, 12, 3736.
DOI: 10.1021/ol101130e)
that Fe could be used to catalyze the rearrangement of the azirine 26 to the
indole 27. It would be interesting to know whether the azirines prepared
(Chem. Commun. 2010, 46, 7993.
DOI: 10.1039/C0CC02863K)
by Qing Lin of the State University of New York at Buffalo will undergo
a similar rearrangement. Mark Lautens of the University of Toronto coupled
(Org. Lett. 2010, 12, 3312.
DOI: 10.1021/ol100975b)
an iodoarene 28 with an azirine 29 under Catellani conditions to give
the indole 30. Christopher J. Moody of the University of Nottingham prepared
(J. Org. Chem. 2010, 75, 6023.
DOI: 10.1021/jo101071c)
the indolequinone 33 by oxidative coupling
of 32 with the benzoquinone 31.
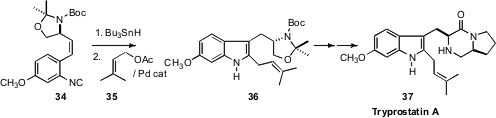
Several years ago, Tohru Fukuyama developed the
Bu3SnH-mediated cyclization of
isonitriles such as 34. He has found
(Angew. Chem. Int. Ed. 2010, 49, 9262.
DOI: 10.1002/anie.201004963)
that the product stannyl indole can be coupled with prenyl acetate 35 under
Pd catalysis. The product 36 was carried on directly to Tryprostatin A (37).