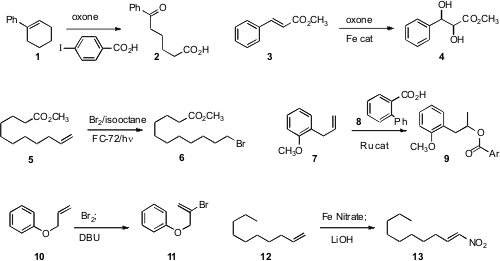
Masahito Ochiai developed
(![]()
2008, March 24) the iodosobenzene-mediated
cleavage of alkenes to keto aldehydes. PMID:23557924 Thottumkara K.
Vinod of Western Illinois University described
(Org. Lett. 2010, 12, 5640.
DOI: 10.1021/ol1023807)
a modified protocol that delivered the keto acid 2.
Chi-Ming Che of the University of Hong Kong established
(J. Am. Chem. 4-Methylbenzenesulfonyl cyanide In stock Soc. 2010, 132, 13229.
DOI: 10.1021/ja100967g)
a method for the preparative scale Fe-catalyzed cis
dihydroxylation of an alkene 3.
Ilhyong Ryu of Osaka Prefecture University devised
(Synlett 2010, 2014.
DOI: 10.1055/s-0030-1258482)
a practical procedure for the free radical addition of HBr to an alkene 5.
Tetsuo Ohta of Doshisha University showed
(Tetrahedron Lett. 1196154-13-8 Data Sheet 2010, 51, 2806.
DOI: 10.1016/j.tetlet.2010.03.052)
that a Ru catalyst could add an aromatic acid to the internal carbon of a terminal alkene 7.
Noriki Kutsumura and Takao Saito of the Tokyo University of Science found
(Org. Lett. 2010, 12, 3316.
DOI: 10.1021/ol101110v)
conditions for bromination/dehydrobromination to convert 10 to 11.
Tsuyoshi Taniguchi of Kanazawa University oxidized
(J. Org. Chem. 2010, 75, 8126.
DOI: 10.1021/jo101769d)
the alkene 12 to the nitro alkene 13.
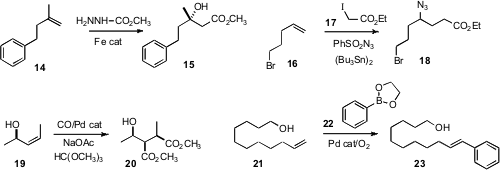
Professor Taniguchi added
(Angew. Chem. Int. Ed. 2010, 49, 10154.
DOI: 10.1002/anie.201005574)
methyl carbazate to 14 to give the β-hydroxy ester 15. Philippe
Renaud of the University of Bern effected
(J. Am. Chem. Soc. 2010, 132, 17511.
DOI: 10.1021/ja1068036)
the free radical homologation of 16 to the azide 18. Daniel P. Becker of Loyola University described
(Tetrahedron Lett. 2010, 51, 3514.
DOI: 10.1016/j.tetlet.2010.04.105)
the elegant diastereoselective Pd-catalyzed bis-methoxycarbonylation of 19
to the diester 20. Matthew S. Sigman of the University of Utah established
(J. Am. Chem. Soc. 2010, 132, 13981.
DOI: 10.1021/ja1060998)
the oxidative Heck arylation of 21 to 23.
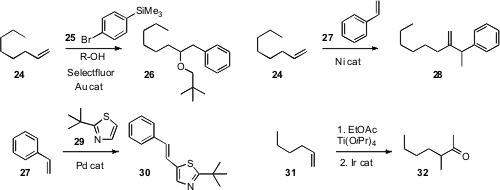
F. Dean Toste of the University of California, Berkeley found
(Org. Lett. 2010, 12, 4728.
DOI: 10.1021/ol102194c)
that the intermediate in the gold-catalyzed alkoxylation of 24
could couple to an aryl silane 25 to give 26.
Chun-Yu Ho of the Chinese University of Hong Kong used
(Angew. Chem. Int. Ed. 2010, 49, 9182.
DOI: 10.1002/anie.201001849)
a Ni catalyst to add styrene 27 to the alkene 24.
Masahiro Miura of Osaka University effected
(J. Org. Chem. 2010, 75, 5421.
DOI: 10.1021/jo101214y)
the oxidative coupling of 29 with styrene
27 to give the linear product 30.
Several years ago
(J. Am. Chem. Soc. 1996, 118, 4198.
DOI: 10.1021/ja954147f),
Jin Kun Cha, now at Wayne State University, reported a modified
Kulinkovich
protocol for coupling an ester with an alkene to give the cyclopropanol. Timothy
W. Funk of Gettysburg College developed
(Tetrahedron Lett. 2010, 51, 6726.
DOI: 10.1016/j.tetlet.2010.10.067)
an Ir catalyst for the specific cleavage of the cyclopropanol,
effecting the net homologation of an alkene 31 to the branched ketone 32.