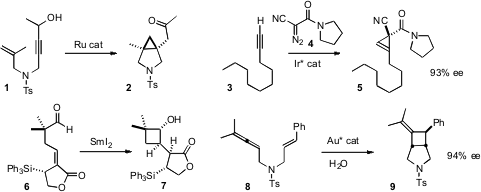
Barry M. 6-Bromo-3-chloroisoquinoline structure Trost of Stanford University generated
(J. Am. Chem. Soc. 2011, 133, 4766.
DOI: 10.1021/ja200971v)
a β-keto carbene from the propargyl alcohol 1, leading to the
cyclopropane
2. Tsutomu Katsuki of Kyushu University devised
(J. Am. Ethyl 4-methyl-1H-pyrrole-2-carboxylate Formula Chem. Soc. 2011, 133, 170.
DOI: 10.1021/ja1089217)
an Ir catalyst for the enantioselective
cyclopropenation of a terminal
alkyne 3, to give 5.
David J. PMID:24059181 Procter of the University of Manchester showed
(Org. Lett. 2010, 12, 5446.
DOI: 10.1021/ol102278c)
that the SmI2-mediated cyclization of 6 proceeded with high
diastereocontrol. F. Dean Toste of the University of California, Berkeley developed
(J. Am. Chem. Soc. 2011, 133, 5500.
DOI: 10.1021/ja200084a)
a gold catalyst for the enantioselective cyclization of 8 to 9.
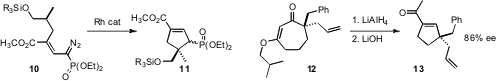
Jon D. Rainier of the University of Utah found
(Org. Lett. 2011, 13, 700.
DOI: 10.1021/ol102931n)
that the readily-prepared diazo ester 10 cyclized smoothly to
11. Brian M. Stoltz of the California Institute of Technology rearranged
(Angew. Chem. Int. Ed. 2011, 50, 2756.
DOI: 10.1002/anie.201007814)
12, prepared by enantioselective allylation, to the
cyclopentene 13.
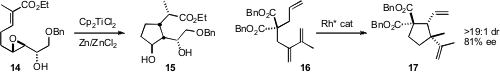
Tushar Kanti Chakraborty of the Indian Institute of Chemical Technology cyclized
(Tetrahedron Lett. 2011, 52, 1709.
DOI: 10.1016/j.tetlet.2011.02.001)
the epoxy ester 14 to the cyclopentanol 15.
Zhi-Xiang Yu of Peking University found
(Angew. Chem. Int. Ed. 2011, 50, 2144.
DOI: 10.1002/anie.201005215)
that a BINOL-derived catalyst cyclized 16 to 17. Related
transition metal-mediated cyclizations, not illustrated, have been reported
(Org. Lett. 2011, 13, 1517,
DOI: 10.1021/ol200157x;
2630,
DOI: 10.1021/ol200741j.)
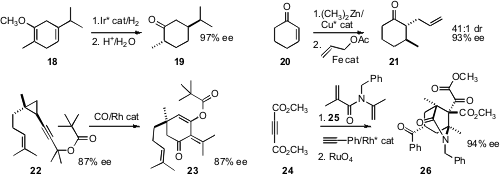
Pher G. Andersson of Uppsala University reduced
(Chem. Commun. 2011, 47, 3989.
DOI: 10.1039/C0CC05619G)
the inexpensive Birch reduction product 18 to give, after hydrolysis, the
cyclohexanone 19 in high ee. Silas P. Cook of the University of Indiana found
(Org. Lett. 2011, 13, 1904.
DOI: 10.1021/ol200059u)
conditions for the allylation of the zinc enolate
resulting from enantioselective
conjugate addition to
cyclohexenone 20. This
approach worked for other ring sizes also.
Weiping Tang of the University of Wisconsin effected
(Angew. Chem. Int. Ed. 2011, 50, 1346.
DOI: 10.1002/anie.201006881)
regioselective cyclocarbonylation of 22 to give the cyclohexanone 23.
Ken Tanaka of the Tokyo University of Agriculture and Technology devised
(Angew. Chem. Int. Ed. 2011, 50, 1664.
DOI: 10.1002/anie.201004150)
a spectacular three-component coupling leading, after oxidative coupling,
to the cyclohexane 26.
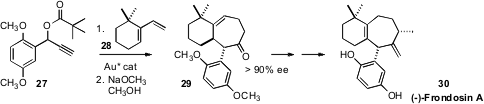
Cristina Nevado of the University of Zurich condensed
(Angew. Chem. Int. Ed. 2011, 50, 911.
DOI: 10.1002/anie.201006105)
27 with 28 to give, after methanolysis, the
cycloheptanone 29 in high ee.
The conversion of 29 to (-)-Frondosin A (30) had already been established.