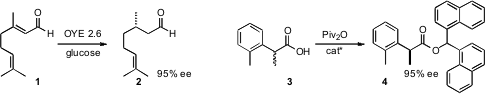
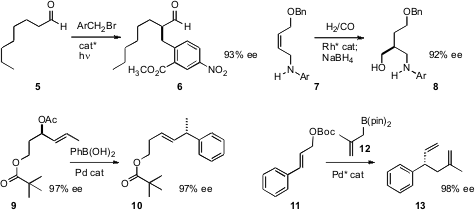
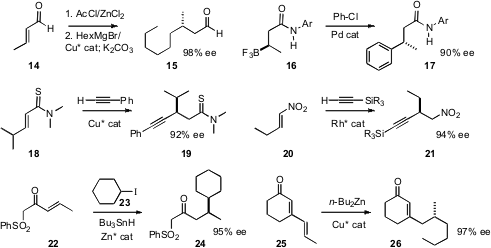
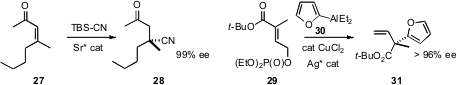
Jon D. Stewart of the University of Florida established
(Chem. Commun. 2010, 46, 8558.
DOI: 10.1039/C0CC03119D)
a scalable enzymatic reduction of geranial 1 to citronellal 2.
Andreas S. Bommarius of Georgia Tech reported
(Chem. Commun. TCEP (hydrochloride) site 2010, 46, 8809.
DOI: 10.1039/C0CC02354J)
related studies. Isamu Shiina of the Tokyo University of Science developed
(J. Am. Chem. Soc. 2010, 132, 11629.
DOI: 10.1021/ja103490h)
a nucleophilic catalyst for the kinetic resolution of α-chiral carboxylic acids such as
3. PMID:35901518
David W. C. MacMillan of Princeton University devised
(J. Am. Chem. Soc. 2010, 132, 13600.
DOI: 10.1021/ja106593m)
a protocol for the enantioselective
benzylation of an aldehyde
5. Kian L. Tan of Boston College
(J. Am. Chem. Soc. 5-Chloroquinolin-8-amine supplier 2010, 132, 14757.
DOI: 10.1021/ja107433h)
and Shannon S. Stahl and Clark R. Landis of the University of Wisconsin
(J. Am. Chem. Soc. 2010, 132, 14027.
DOI: 10.1021/ja106674n)
developed the regioselective enantioselective hydroformylation of alkenes such as 7 with chelating substituents.
Masaya Sawamura of Hokkaido University
(J. Am. Chem. Soc. 2010, 132, 879.
DOI: 10.1021/ja9092264)
and others
(Org. Lett. 2010, 12, 2438,
DOI: 10.1021/ol100841y;
Tetrahedron Lett. 2010, 5592,
DOI: 10.1016/j.tetlet.2010.08.051;
6018, DOI: 10.1016/j.tetlet.2010.09.035) effected
enantiospecific allylic coupling, as in the conversion of 9 to 10. James P. Morken, also of Boston College achieved
(J. Am. Chem. Soc. 2010, 132, 10686.
DOI: 10.1021/ja105161f)
enantioselective allylation of 11.
Ben L. Feringa of the University of Groningen devised
(J. Am. Chem. Soc. 2010, 132, 13152.
DOI: 10.1021/ja105585y)
a protocol for net enantioselective
conjugate addition to an α, β-unsaturated aldehyde 14.
Gary A. Molander of the University of Pennsylvania found
(J. Am. Chem. Soc. 2010, 132, 17108.
DOI: 10.1021/ja108949w)
that coupling of 16, prepared by enantioselective
conjugate addition, proceeded with inversion. Naoya Kumagai and Masakatsu
Shibasaki of the Institute of Microbial Chemistry effected
(J. Am. Chem. Soc. 2010, 132, 10275.
DOI: 10.1021/ja105141x)
enantioselective alkynylation of the thioamide 18, and Takahiro
Nishimura and Tamio Hayashi of Kyoto University achieved
(Chem. Commun. 2010, 46, 6837.
DOI: 10.1039/C0CC02181D)
conjugate alkynylation of the nitroalkene 20. Several other protocols
(Angew. Chem. Int. Ed. 2010, 49, 5780,
DOI: 10.1002/anie.201001883;
7299, DOI: 10.1002/anie.201003172;
8145, DOI: 10.1002/anie.201004980;
J. Am. Chem. Soc. 2010, 132, 14373,
DOI: 10.1021/ja106809p;
J. Org. Chem. 2010, 75, 7829.
DOI: 10.1021/jo101704b)
have been developed for the catalytic enantioselective construction of arylated stereogenic centers.
Sunggak Kim, now at Nanyang Technological University, showed
(Tetrahedron Lett. 2010, 51, 4947.
DOI: 10.1016/j.tetlet.2010.07.014 )
that free radical addition to 22 could be effected with
high ee. The product 26 from the enantioselective 1,6-addition developed
(Org. Lett. 2010, 12, 4335.
DOI: 10.1021/ol1017382)
by Alexandre Alexakis of the Université de Genève and
Christophe Crévisy and Marc Mauduit of ENSC Rennes is interesting in itself, and
could also be epoxidized and cleaved by the Eschenmoser protocol to deliver the
alkynyl ketone.
To prepare acyclic alkylated quaternary centers, Professor Shibasaki and
Motomu Kanai of the University of Tokyo developed
(J. Am. Chem. Soc. 2010, 132, 8862.
DOI: 10.1021/ja1035286)
a Sr catalyst for the conjugate addition of cyanide, and Amir H. Hoveyda,
also of Boston College, achieved
(Angew. Chem. Int. Ed. 2010, 49, 8370.
DOI: 10.1002/anie.201005124) the
allylic coupling of 29 with 30.