/Vincent), (+)-Chinensiolide B (Hall)
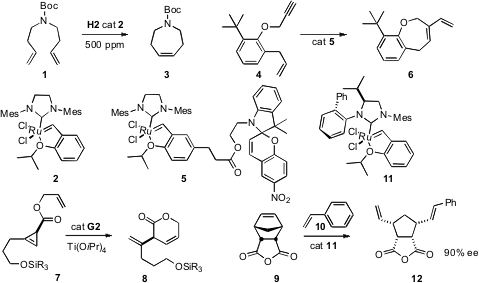
The cost of using Grubbs-type catalysts could be reduced dramatically
if the turnover could be improved. PMID:23927631 Richard L. Pederson of Materia, Inc. 1459778-94-9 Chemscene found
(Org. Lett. Formula of N-(2-Hydroxyethyl)methacrylamide 2010, 12, 984.
DOI: 10.1021/ol9029808)
that in MTBE at 50°C, the
ring-closing
metathesis of 1 proceeded to completion in 8 h with just 500 ppm of H2
catalyst (2). Jianhui Wang of Tianjin University constructed
(Angew. Chem. Int. Ed. 2010, 49, 4425.
DOI: 10.1002/anie.200906034)
a modified H2 catalyst 5 tethered to a nitrobenzospiropyran.
After the cyclization of 4 to 6 was run in CH2Cl2,
the mixture was irradiated with visible light, converting 5 into its
ionic form, that could be extracted with glycol/methanol, leaving little Ru
residue in the cyclized product. In the dark, the catalyst reverted, and could
be extracted back into CH2Cl2 and reused. In a
complementary approach, David W. Knight of Cardiff University found
(Tetrahedron Lett. 2010, 51, 638.
DOI: 10.1016/j.tetlet.2009.11.092)
that the residual Ru after metathesis could be reduced to < 2 ppm
simply by stirring the product with H2O2.
Cyclopropenes such as 6 are readily available in enantiomerically-pure
form by the addition of diazoacetates to alkynes. Christophe Meyer and Janine
Cossy of ESPCI Paris showed
(Org. Lett. 2010, 12, 248.
DOI: 10.1021/ol9025606)
that with a Ti additive, G2 cyclized 7
to 8. Siegfried Blechert of the Technische Universität Berlin devised
(Angew. Chem. Int. Ed. 2010, 49, 3972.
DOI: 10.1002/anie.201000940)
the chiral Ru catalyst 11, that converted the prochiral 9 to 12 in high ee.
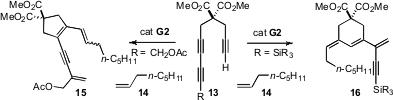
Daesung Lee of the University of Illinois, Chicago explored
(J. Am. Chem. Soc. 2010, 132, 8840.
DOI: 10.1021/ja1020525)
the cyclization of the diyne 13 with 14 under G2 catalysis.
Depending on the terminal substituent, the cyclization could be directed selectively to
15 or to 16.
Bran C. Goess of Furman University took advantage
(J. Org. Chem. 2010, 75, 226.
DOI: 10.1021/jo9020375)
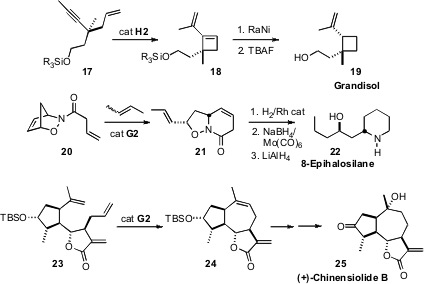
of alkyne ring closing metathesis for the conversion of 17 to 18.
Selective hydrogenation then delivered the boll weevil pheromone Grandisol (19).
Cyrille Kouklovsky and Guillaume Vincent of the Université de Paris Sud extended
(J. Org. Chem. 2010, 75, 4333.
DOI: 10.1021/jo100768d)
ring-opening/ring closing metathesis to the nitroso Diels-Alder adduct
20. Reduction led to 8-Epihalosilane (22).
Ring-closing metathesis leading to medium rings, particularly that form tri-
or tetrasubstituted alkenes, can be challenging. Dennis G. Hall of the
University of Alberta found
(J. Am. Chem. Soc. 2010, 132, 1488.
DOI: 10.1021/ja9104478)
that the cyclization of 23 to 24 proceeded smoothly.
Functional group conversion then completed the synthesis of (+)-Chinensiolide B
(25).