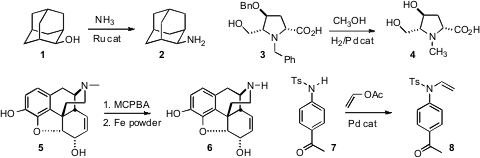
There have been several significant advances in N-alkylation using alcohols.
Matthias Beller of Universität Rostock devised
(Angew. PMID:24202965 Chem. Int. Ed. 2010, 49, 8126.
DOI: 10.1002/anie.201002576)
a Ru catalyst for the amination of secondary and benzylic primary alcohols with
ammonia. Dieter Vogt of the Eindhoven University of Technology reported
(Angew. Chem. Int. Ed. 2010, 49, 8130.
DOI: 10.1002/anie.201002583)
related transformations. Pei-Qiang Huang of Xiamen University showed
(Chem. Commun. 2010, 46, 7834.
DOI: 10.1039/C0CC01487G)
that debenzylation of 3 in methanol led to the N-methyl amine 4.
Parallel results have been reported with Ir
(J. Am. Chem. Fmoc-N-Me-Phe-OH Order Soc. 2010, 132, 15108.
DOI: 10.1021/ja107274w),
Au (Chem. Eur. J. 2010, 16, 13965.
DOI: 10.1002/chem.201001848)
and Cu (Chem. Lett. 2010, 39, 1182.
DOI: 10.1246/cl.2010.1182).
Peter J. Scammells of Monash University found
(J. Org. Chem. 2409005-96-3 supplier 2010, 75, 4806.
DOI: 10.1021/jo1008492)
that demethylation of an N-oxide could be effected with Fe powder. Yao Fu and
Qingxiang Guo of the University of Science and Technology of China N-vinylated
(Tet. Lett. 2010, 51, 5476.
DOI: 10.1016/j.tetlet.2010.08.029)
a sulfonamide 7 with vinyl acetate and a Pd catalyst. Acyl amides
could also be N-vinylated under these conditions.
Hirokazu Urabe of the Tokyo Institute of Technology reported
(Org. Lett. 2010, 12, 4137.
DOI: 10.1021/ol101541p)
that the stereodefined secondary sulfonamide of 9 could
be displaced by an internal nucleophile, to give the product 11
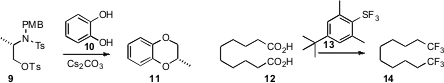
with inversion of absolute configuration. Teruo Umemoto of IM&T Research devised
(J. Am. Chem. Soc. 2010, 132, 18199.
DOI: 10.1021/ja106343h)
the remarkable fluorinating agent 13. In addition to converting secondary alcohols to
the corresponding fluorides and ketones to gem-difluorides, 13 cleanly
converted the carboxylic acids of 12 to trifluoromethyl groups.
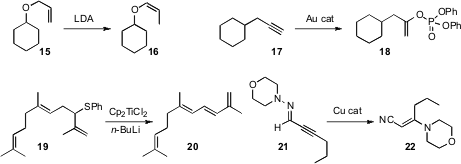
Paul G. Williard of Brown University demonstrated
(Org. Lett. 2010, 12, 5378.
DOI: 10.1021/ol102029u)
that LDA converted an allyl ether 15 specifically to the
(Z)-propenyl ether 16. Phil Lee Ho of Kangwon National University
and Sunggak Kim of Nanyang Technological University could add
(Angew. Chem. Int. Ed. 2010, 49, 6806.
DOI: 10.1002/anie.201001799)
a phosphate to an alkyne 17 to make either the less substituted or
the more substituted enol phosphate. Professor Kim reported
(J. Org. Chem. 2010, 75, 7928.
DOI: 10.1021/jo101543q)
similar results with the addition of carboxylic acids.
Nigel Ribeiro of the Université de Strasbourg effected
(Synlett 2010, 2928.
DOI: 10.1055/s-0030-1259009)
smooth elimination of the allylic thioether 19 to the
triene 20. Itaru Nakamura of Tohoku University found
(Org. Lett. 2010, 12, 4198.
DOI: 10.1021/ol1017504)
that a hydrazone 21 could be rearranged to the nitrile 22.
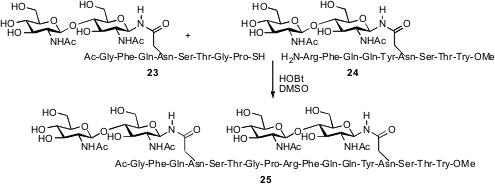
Samuel J. Danishefsky of Sloan-Kettering devised
(J. Am. Chem. Soc. 2010, 132, 17045.
DOI: 10.1021/ja1084628)
conditions for coupling the glycopeptides 23
and 24. Remarkably, the assembly of 25 was successful even with
unprotected hydroxyl groups on the sugars and on the peptide.