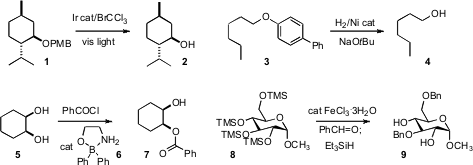
Corey R. J. Stephenson of Boston University devised
(Chem. Commun. 2011, 47, 5040.
DOI: 10.1039/C1CC10827A)
a protocol using visible light for removing the PMB group from 1 to give
2. PMID:23819239 John F. Hartwig, now at the University of California, Berkeley developed
(Science 2011, 332, 439.
DOI: 10.1126/science.1200437)
a Ni catalyst for the cleavage of the durable aryl
ether of 3 to give 4. Mark S. Dibenzyl carbonate Chemical name Taylor of the University of Toronto devised
(J. 774212-81-6 site Am. Chem. Soc. 2011, 133, 3724.
DOI: 10.1021/ja110332r)
the catalyst 6, that selectively mediated
esterification of 5 to 7.
Jean-Marie Beau of the Université Paris-Sud added
(Chem. Commun. 2011, 47, 2146.
DOI: 10.1039/C0CC04398B)
Et3SiH following the Fe-catalyzed deprotection-protection of 8,
resulting in clean conversion to the bis
benzyl ether 9.
Mahmood Tajbakhsh of the University of Mazandaran showed
(Tetrahedron Lett. 2011, 52, 1260.
DOI: 10.1016/j.tetlet.2011.01.023)
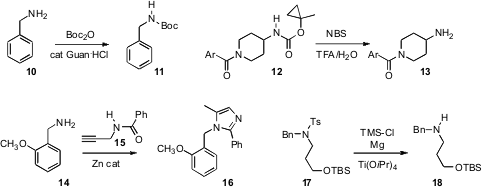
that guanidine HCl catalyzed the conversion of 10 to 11. Stephen W. Wright
of Pfizer/Groton established
(Tetrahedron Lett. 2011, 52, 3171.
DOI: 10.1016/j.tetlet.2011.04.027)
that the new urethane protecting group of 12, stable to many conditions, could be
deprotected to 13 under conditions that spared even a
Boc group. Matthias
Beller of the Leibniz-Institute for Catalysis protected
(Chem. Commun. 2011, 47, 2152.
DOI: 10.1039/C0CC04625F)
the amine 14 as the readily-hydrolyzed imidazole 16.
Sentaro Okamoto of Kanagawa University found
(Org. Lett. 2011, 13, 2626.
DOI: 10.1021/ol200740r)
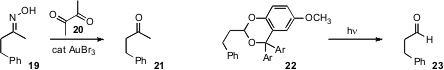
a simple reagent combination for the removal
of the sometimes reluctant
sulfonamide from 17.
Jordi Burés and Jaume Vilarrasa of the Universitat de Barcelona removed
(Angew. Chem. Int. Ed. 2011, 50, 3275.
DOI: 10.1002/anie.201007269)
the oxime from 19 by Au-catalyzed exchange with
20. Pengfei Wang of the University of Alabama, Birmingham designed
(J. Org. Chem. 2011, 76, 2040.
DOI: 10.1021/jo102429g)
a range of photochemically-removable protecting groups for aldehydes and ketones.
Rafael Robles of the University of Granada selectively protected
(J. Org. Chem. 2011, 76, 2277.
DOI: 10.1021/jo102395c)
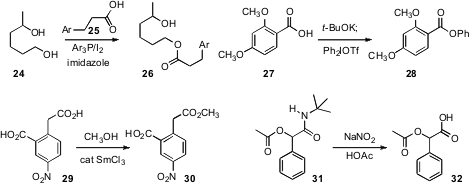
the diol 24 using the reagent created by the activation of
25. Berit Olofsson of Stockholm University prepared
(Org. Lett. 2011, 13, 3462.
DOI: 10.1021/ol2012082)
the phenyl ester 28 by exposing 27 to the diaryl iodonium triflate. Kannoth
Manheri Muraleedharan of the Indian Institute of Technology, Madras selectively
(Org. Lett. 2011, 13, 1932.
DOI: 10.1021/ol200247c)
esterified 29 to 30 with catalytic SmCl3.
Amides are usually more resistant than esters to hydrolysis. Bruce Ganem of
Cornell University removed
(Tetrahedron Lett. 2011, 52, 2209.
DOI: 10.1016/j.tetlet.2010.11.156)
the t-butyl amide of 31 in the presence of the usually much more
fragile acetate, to deliver 32.