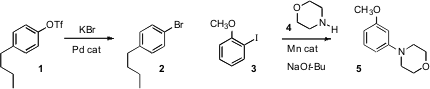
Stephen L. Buchwald of MIT established
(J. Am. Buy885588-14-7 Chem. Soc. 2010, 132, 14076.
DOI: 10.1021/ja107481a)
a Pd-catalyzed protocol for conversion of an aryl triflate 1 to the
halide 2. Jie Wu of Fudan University prepared
(Tetrahedron Lett. 2010, 51, 6646.
DOI: 10.1016/j.tetlet.2010.10.054)
aromatic halides from the corresponding carboxylic acids.
Yong-Chua Teo of Nanyang Technological University described
(Tetrahedron Lett. 2010, 51, 3910.
DOI: 10.1016/j.tetlet.2010.05.098)
the Mn-mediated conversion of 3 to 5, suggesting a benzyne
intermediate. Takanori Shibata of Waseda University effected
(Synlett 2010, 2601.
DOI: 10.1055/s-0030-1258774)
the direct Ru-mediated coupling of aryl halides with amines,
and Paul Helquist of the University of Notre Dame prepared
(J. PMID:23381601 Org. 6-Chlorobenzo[a]phenazin-5-ol Chemscene Chem. 2010, 75, 4887.
DOI: 10.1021/jo101002p)
anilines by coupling aryl halides with NaN3.
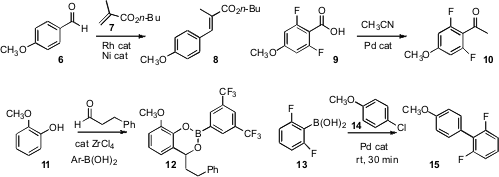
Chao-Jun Li of McGill University devised
(Tetrahedron Lett. 2010, 51, 5486.
DOI: 10.1016/j.tetlet.2010.08.040)
the Pd-catalyzed decarbonylative
Heck coupling of 6 with 7
to give 8. Mats Larhed of Uppsala University showed
(Angew. Chem. Int. Ed. 2010, 49, 7733.
DOI: 10.1002/anie.201003009)
that Pd could also catalyze the decarboxylative coupling of an
aromatic acid 9 with a nitrile to give the ketone 10.
Dennis G. Hall of the University of Alberta found
(Tetrahedron Lett. 2010, 51, 4256.
DOI: 10.1016/j.tetlet.2010.06.035)
that an areneboronic acid could promote the Zr-catalyzed ortho condensation
of a phenol 11 with an aldehyde, leading to 12, that could
then be carried on to a range of other products. Professor Hall also showed
(Angew. Chem. Int. Ed. 2010, 49, 2883.
DOI: 10.1002/anie.200906710)
that areneboronic acids are stable to many standard organic
transformations, and that the product boronic acids can be readily purified by
extraction into sorbitol/Na2CO3. Professor Buchwald reported
(J. Am. Chem. Soc. 2010, 132, 14073.
DOI: 10.1021/ja1073799)
an optimized source of Pd for the
Suzuki-Miyaura coupling, allowing the room temperature
participation even of unstable boronic acids such as 13.
Wing-Yiu Yu of the Hong Kong Polytechnic University observed
(J. Am. Chem. Soc. 2010, 132, 12862.
DOI: 10.1021/ja106364r)
that 17 was an effective donor for the Pd-catalyzed ortho C-H amination
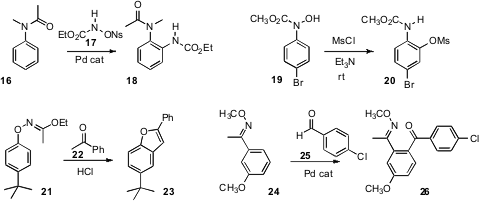
of 16. Nicholas C. O. Tomkinson of Cardiff University uncovered
(Synlett 2010, 2471.
DOI: 10.1055/s-0030-1258546)
the facile rearrangement of 19 to 20.
Professor Buchwald described
(J. Am. Chem. Soc. 2010, 132, 9990.
DOI: 10.1021/ja1044874)
the coupling of 21, prepared from the aryl halide, with 22
to give the benzofuran 23. Professor Yu devised
(Org. Lett. 2010, 12, 3926.
DOI: 10.1021/ol101618u)
the Pd-catalyzed ortho C-H coupling of 24 with
an aldehyde 25 to give the ketone 26.
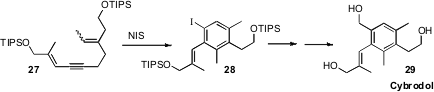
For highly-substituted benzene derivatives, it is sometimes more effective to
construct the benzene ring. In the course of a synthesis of Cybrodol (29),
Stefan F. Kirsch of the Technische Universität München cyclized
(Angew. Chem. Int. Ed. 2010, 49, 4661.
DOI: 10.1002/anie.201001113)
the alkyne 27 to 28 with
N-iodosuccinimide.