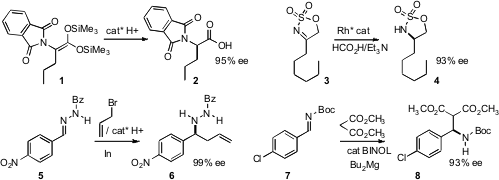
Takashi Ooi of Nagoya University effected
(J. Am. Chem. PMID:23847952 Soc. 2010, 132, 12240.
DOI: 10.1021/ja105945z)
the enantioselective protonation of ketene silyl acetals such as 1 to give 2
in high ee. Hyeon-Kyu Lee of the Korean Research Institute of Chemical Technology achieved
(Org. Lett. 2010, 12, 4184.
DOI: 10.1021/ol1017905)
high ee in the hydrogenation of the cyclic sulfamidate 3 to 4. Doo Ok Jang of Yonsei University combined
(J. Am. 99116-11-7 custom synthesis Chem. 1639-66-3 site Soc. 2010, 132, 12168.
DOI: 10.1021/ja1035336)
the nucleophilic allyl indium with a protonated chiral amine to effect homologation of 5
to 6 [this result has been retracted (
J. Am. Chem. Soc. 2014, 136, 11850.
DOI: 10.1021/ja1035336)].
Ryo Shintani and Tamio Hayashi of Kyoto University reported
(Org. Lett. 2010, 12, 4106.
DOI: 10.1021/ol101700v)
a related advance with tetraarylborates. Kazuaki Ishihara, also of Nagoya University
(Org. Lett. 2010, 12, 3502.
DOI: 10.1021/ol101353r)
and Yoshihiro Sohtome and Kazuo Nagasawa of the Tokyo University of Agriculture and Technology
(Angew. Chem. Int. Ed. 2010, 49, 9254.
DOI: 10.1002/anie.201005109)
devised conditions for adding malonate to imines such as 7.
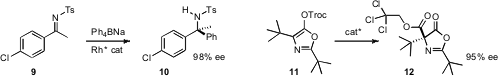
Professors Shintani and Hayashi also employed
(J. Am. Chem. Soc. 2010, 132, 13168.
DOI: 10.1021/ja106114q)
tetraarylborates to convert 9 to the α-quaternary amine 10. Professor Ooi
(Angew. Chem. Int. Ed. 2010, 49, 5567.
DOI: 10.1002/anie.201005109)
and Wanbin Zhang of Shanghai Jiao Tong University
(J. Am. Chem. Soc. 2010, 132, 15939.
DOI: 10.1021/ja109069k)
prepared α-quaternary amino acids such as 12 by nucleophilic rearrangement of 11.
Keiji Maruoka, also of Kyoto University, reported
(J. Am. Chem. Soc. 2010, 132, 17074, not illustrated,
DOI: 10.1021/ja107897t)
a catalytic enantioselective
conjugate addition approach to α-quaternary amines.
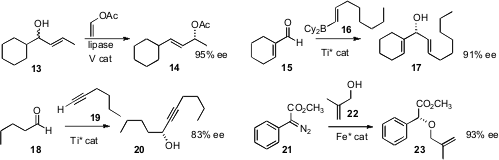
Shuji Akai of the University of Shizuoka converted
(Org. Lett. 2010, 12, 4900.
DOI: 10.1021/ol102053a)
the racemic allylic alcohol 13 to the enantiomerically-enriched acetate
14 by combining V-catalyzed equilibration with lipase-catalyzed acylation. Toshiro
Harada of the Kyoto Institute of Technology added
(Org. Lett. 2010, 12, 5270.
DOI: 10.1021/ol1023213)
the alkenylboron 16 to the aldehyde 15 with high ee. Xiang Zhou of Wuhan
University and Lin Pu of the University of Virginia significantly improved
(Tetrahedron Lett. 2010, 51, 5024.
DOI: 10.1016/j.tetlet.2010.07.082)
a protocol for the enantioselective addition of aliphatic
alkynes to aliphatic aldehydes. (For other enantioselective additions to
aldehydes, not illustrated, see
J. Org. Chem. 2010, 75, 5326,
DOI: 10.1021/jo100674f
and Org. Lett. 2010, 12, 5088,
DOI: 10.1021/ol102234k).
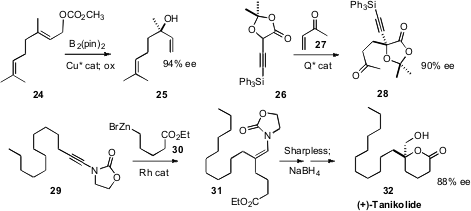
Qi-Lin Zhou of Nankai University described
(Nature Chem. 2010, 2, 546.
DOI: 10.1038/nchem.651)
the remarkable Fe-catalyzed coupling of the diazo ester 21 with an
alcohol 22 to give the ether 23 in high ee. Silanes
(J. Am. Chem. Soc. 2010, 132, 9289.
DOI: 10.1021/ja103747h)
and amines
(Angew. Chem. Int. Ed. 2010, 49, 4763.
DOI: 10.1002/anie.201001686)
have also been prepared in high ee from diazo esters.
Amir H. Hoveyda of Boston College
(J. Am. Chem. Soc. 2010, 132, 10630,
DOI: 10.1021/ja104777u, 10634,
DOI: 10.1021/ja104254d)
and others
(Chem. Eur. J. 2010, 16, 13609,
DOI: 10.1002/chem.201002361;
Org. Lett. 2010, 12, 4098,
DOI: 10.1021/ol101691p)
have described enantioselective conjugate and allylic borylation, exemplified by the
conversion of 24 to 25. Professor Maruoka effected
(Chem. Commun. 2010, 46, 7593.
DOI: 10.1039/C0CC02334E)
catalytic enantioselective alkylation and conjugate addition of 26.
Hon Wai Lam of the University of Edinburgh showed
(Angew. Chem. Int. Ed. 2010, 49, 8733.
DOI: 10.1002/anie.201004328)
that the trisubsituted alkene 31 could be dihydroxylated using
the improved Sharpless protocol. This enabled the straightforward construction
of the antifungal lactone (+)-Tanikolide (32).