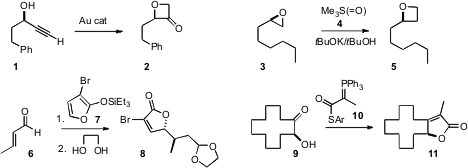
Liming Zhang of the University of California, Santa Barbara described
(J. Am. Chem. Soc. (R)-2-amino-1-phenylethan-1-ol manufacturer 2010, 132, 8550.
DOI: 10.1021/ja1033952)
the remarkable transformation of a propargyl alcohol 1
into the oxetanone 2. The transformation proceeded without
loss of ee, as did the ring expansion of 3 to 5 reported
(J. Org. Chem. 2010, 75, 6229.
DOI: 10.1021/jo101330p)
by Peter R. 36294-24-3 Purity Schreiner of Justus-Liebig University, Giessen and
Andrey A. Fokin of the Kiev Polytechnic Institute.
Takeo Taguchi of the Tokyo University of Pharmacy and Life Sciences developed
(Chem. Commun. PMID:23310954 2010, 46, 8728.
DOI: 10.1039/C0CC02438D)
a catalyst for the stereoselective conjugate addition of 7 to 6.
Mitsuru Shindo of Kyushu University devised
(Org. Lett. 2010, 12, 5346.
DOI: 10.1021/ol102407k)
the thioester 10, that condensed smoothly with an α-hydroxy ketone
9 to deliver the lactone 11.
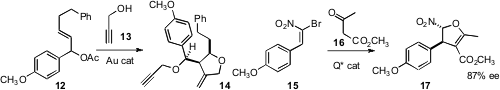
Zili Chen of the Renmin University of China and Lin Guo of the Beijing
University of Aeronautics and Astronautics developed
(Org. Lett. 2010, 12, 3468.
DOI: 10.1021/ol1012923)
the diastereoselective double addition of propargyl alcohol 13 to
12 to give 14.
Jian-Wu Xie of Zhejiang Normal University uncovered
(J. Org. Chem. 2010, 75, 8716.
DOI: 10.1021/jo101935k)
the catalyzed enantioselective addition of 16 to 15 to give the
dihydrofuran 17.
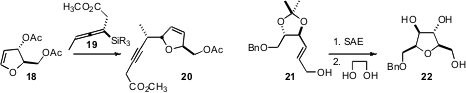
James S. Panek of Boston University extended
(Org. Lett. 2010, 12, 4624.
DOI: 10.1021/ol1019629)
the utility of the enantiomerically-pure allenic nucleophile 19, adding it to
the acceptor 18 to give 20 with both ring and sidechain stereocontrol.
Biswanath Das of the Indian Institute of Chemical Technology, Hyderabad showed
(Tetrahedron Lett. 2010, 51, 6011.
DOI: 10.1016/j.tetlet.2010.09.049)
that the epoxide of the tartrate-derived
acetonide 21 could be
rearranged to the fully-substituted, differentially-protected
tetrahydrofuran 22.
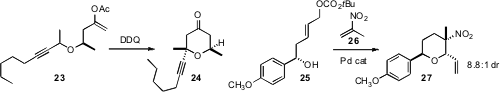
Paul E. Floreancig of the University of Pittsburgh uncovered
(Angew. Chem. Int. Ed. 2010, 49, 5894.
DOI: 10.1002/anie.201002281)
the highly stereocontrolled oxidative cyclization of 22
to 23. Dirk Menche of the University of Heidelberg found
(Angew. Chem. Int. Ed. 2010, 49, 9270.
DOI: 10.1002/anie.201003304)
that the Pd-mediated addition of 24 to 25 also proceeded with high diastereocontrol.
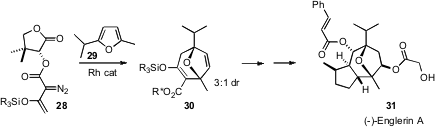
Dipolar cycloaddition to a furan is of increasing importance in
target-directed synthesis. Emmanuel A. Theodorakis of the University of
California, San Diego added
(Org. Lett. 2010, 12, 3708.
DOI: 10.1021/ol1015652)
the diazo ester 27, prepared from the inexpensive chiral auxiliary pantolactone,
to the furan 28. The product diastereomers were readily separated, enabling
efficient access to the potent antineoplastic agent (-)-Englerin A (30).