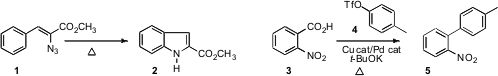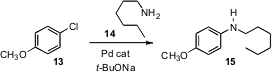Carrying out organic synthesis with a
flow reactor can offer significant
advantages over the more conventional batch processing. Andreas Kirschning of
Leibniz Universität Hannover concisely summarized
(Chem. Commun. 2011, 47, 4583.
DOI: 10.1039/C0CC05060A)
the issues surrounding flow methods, both micro and meso.
Walter Leitner of RWTH Aachen focused
(Chem. Commun. 2011, 47, 3691.
DOI: 10.1039/C0CC04958A)
on near- and supercritical fluids as solvents, and Steven V. Ley of the
University of Cambridge discussed both in-line IR monitoring for the accurate
dispensing of reagents in a flow apparatus
(Chem. Sci. 2011, 2, 765.
DOI: 10.1039/C0SC00603C),
and cryogenic operations
(Org. Lett. 2011, 13, 3312.
DOI: 10.1021/ol2010006). PMID:23341580
Nicholas E. Leadbeater of the University of Connecticut addressed
(Tetrahedron Lett. 2011, 52, 263.
DOI: 10.1016/j.tetlet.2010.11.027)
the handling of solid reaction products, and Thomas Wirth of Cardiff University
(Angew. Chem. Int. Ed. 2011, 50, 357.
DOI: 10.1002/anie.201006107)
and Martyn Poiakoff of the University of Nottingham
(Angew. Chem. Int. Ed. 175281-76-2 web 2011, 50, 3788.
DOI: 10.1002/anie.201100412)
outlined software-based reaction optimization. A recent monograph
(reviewed in J. Am. Chem. Soc. 3-Chloro-5-nitro-1H-pyrazole Order 2011, 133, 9948.
DOI: 10.1021/ja204803k)
by Charlotte Wiles of Chemtrix BV and Paul Watts
of the University of Hull provides a detailed overview of many of these issues.
Simple thermal reactions are easily carried out under flow conditions, with optimized
temperature and dwell times. Peter H. Seeberger of Max Planck Potsdam carried out
(Chem. Commun. 2011, 47, 2688.
DOI: 10.1039/C0CC04481D)
the Hemetsberger-Knittel cyclization of 1 to the indole 2, and Lukas
J. Goossen of TU Kaiserslautern and Toby Underwood of Pfizer/Sandwich effected
(Chem. Commun. 2011, 47, 3628.
DOI: 10.1039/C0CC05708H)
the decarboxylative coupling of 3 with 4 to give 5.
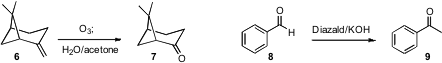
A flow apparatus can also be used for gas-liquid reactions. C. Oliver Kappe
of Karl-Franzen University Graz effected
(Org. Lett. 2011, 13, 984.
DOI: 10.1021/ol102984h)
ozonolysis of 6, using the Dussault protocol, and Dong-Pyo Kim of
Chungnam National University generated
(Angew. Chem. Int. Ed. 2011, 50, 5952.
DOI: 10.1002/anie.201101977)
diazomethane in situ to homologate 8 to 9.
Mixing can be a serious issue under flow conditions.
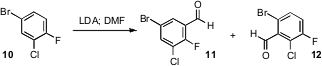
Sarah J. Dolman of Merck Process observed
(J. Org. Chem. 2011, 76, 993.
DOI: 10.1021/jo102275n)
that kinetic deprotonation and formylation of 10 gave 11, but that formylation
after aging led to increasing quantities of 12. Using magnetically-driven
agitation in a tube mixer, she was able to make 11 the dominant product
from the flow procedure.
In converting the coupling of 13 with 14 to flow conditions,
Klavs F. Jensen and Stephen L. Buchwald of MIT had to deal
(Chem. Sci. 2011, 2, 287.
DOI: 10.1039/C0SC00524J)
with the by-product inorganic solids that were produced
in the course of the reaction. They found that maintaining the reaction zone at
60°C in an ultrasonic bath minimized the precipitate bridging that led to
blockage.
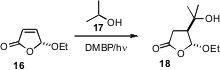
Photochemical reactions have been difficult to scale. With a flow apparatus, as described
(Tetrahedron Lett. 2011, 52, 278.
DOI: 10.1016/j.tetlet.2010.11.018)
by Michael Oelgemöller of James Cook University, transformations such as the addition of
17 to 16 can be facile. The simple apparatus consisted of PTFE
capillary (transparent to UV) wrapped around a light source.
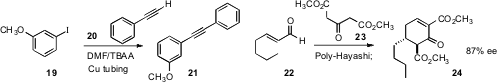
Flow reactors work particularly well with solid catalysts. Sejal Patel and
Nello Mainolfi of Novartis/Cambridge observed
(Org. Lett. 2011, 13, 280.
DOI: 10.1021/ol1026848)
that the Castro-Stephens coupling of 19 with 20 could
be effected by flowing the reactants through heated copper tubing. Miquel A.
Pericàs of ICIQ Tarragona and the Universitat de Barcelona found
(Synlett 2011, 464.
DOI: 10.1055/s-0030-1259528)
that an immobilized Hayashi catalyst functioned well for the
enantioselective Robinson annulation of 23 with 22.