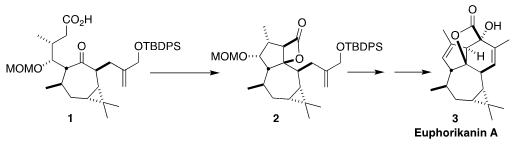
Euphorikanin A (3), isolated from the roots of the Chinese medicinal herb
Euphorbia kansui, showed the ability to reactivate latent HIV. En route to
3, Yanxing Jia of Peking University cyclized the keto acid 1 to the β-lactone
2
(Angew. Chem. PMID:23775868 Price of (S)-(-)-tert-Butylsulfinamide Price of tert-Butyl 2-diazoacetate Int. Ed. 2022, 61, e202200576.
DOI: 10.1002/anie.202200576).
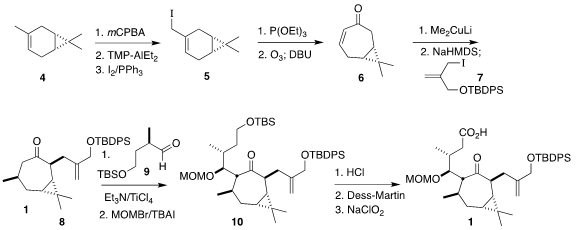
The starting material for the synthesis was the inexpensive carene 4. In the first stage, the conversion of
4 to the iodide 5 was developed, leading to
significantly improved production of the previously-known
cycloheptenone
6.
Conjugate addition of Me2CuLi proceeded across the exo face of the enone, to
give a ketone that was kinetically deprotonated, then alkylated with the iodide
7, completing the assembly of 8.
Aldol reaction with the aldehyde
9 followed
by protection led to 10, that was deprotected and oxidized to the keto acid
1.
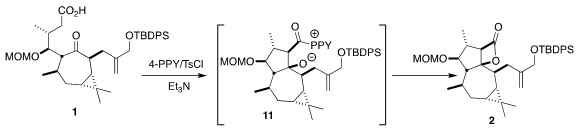
Following the Romo protocol, 1 was cyclized to the
β-lactone 2. This
cyclization probably proceeds via intramolecular aldol reaction of the mixed
anhydride, leading to 11.
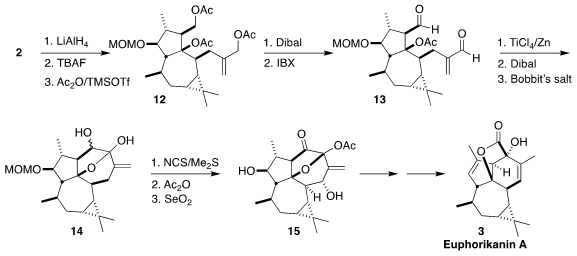
Reduction of 2 followed by
desilylation led to the triol, the tertiary
alcohol of which was prone to dehydration. Eventually conditions were found that
allowed peracetylation, to give 12. Selective reduction followed by oxidation
led to the dialdehyde, that was cyclized to the diol using the
McMurry conditions. Dibal deprotected the tertiary acetate, and the mixture of triols
was selectively oxidized, leading to 14 as a mixture of diastereomers.
Corey-Kim oxidation to the diketone led to the hemiketal, that was protected and
oxidized to give 15. Dehydration followed by allylic reduction and
benzylic acid
rearrangement then completed the synthesis of euphorikanin A (3).
It is instructive to compare the approach to euphorikanin A (3) outlined here
to the route described by Carreira
(![]() 2022, January 3).
2022, January 3).