(+)-Subincanadine F (Li), (±)-Strychnine (Reissig), (-)-Virginiamycin M2 (Panek)
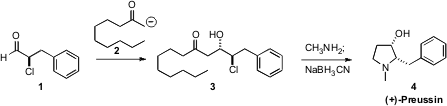
Aldehydes such as 1 are readily available by direct enantioselective
chlorination. Robert Britton of Simon Fraser University found
(Org. Lett. 2010, 12, 4034.
DOI: 10.1021/ol101631e)
that the addition of the kinetic ketone enolate
2 gave the anti aldol 3. Condensation of the chlorohydrin 3
with a primary amine led to the cyclic pyrrolinium salt, that was reduced with
high diastereocontrol to (+)-Preussin (4). 4-(1H-Benzimidazol-2-yl)benzoic acid structure
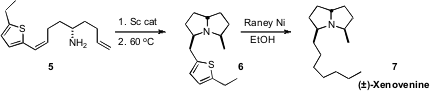
Tom Livinghouse of Montana State University developed Sc catalysts for the
cyclization of γ-amino terminal alkenes such as 5. PMID:28630660 In contrast, addition to
internal alkenes was sluggish. BuyChloroiridic acid He has now shown
(Org. Lett. 2010, 12, 4271.
DOI: 10.1021/ol101646t)
that a thiophene substituent activated the internal alkene for addition, enabling the
facile synthesis of (±)-Xenovenine (7).
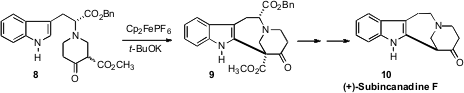
Chaozhong Li of the Shanghai Institute of Organic Chemistry found
(Chem. Commun. 2010, 46, 8436.
DOI: 10.1039/C0CC03428B)
that ferrocenium ion cleanly oxidized the enolate of the
β-keto ester 8, effecting cyclization to 9. The D-tryptophan-derived ester that
directed the relative and absolute configuration of the cyclization could
readily by removed, delivering (+)-Subincanadine F (10).
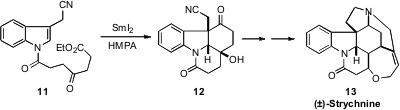
In a complementary approach to
indole alkaloid synthesis, Hans-Ulrich Reissig
of the Freie Universität Berlin devised
(Angew. Chem. Int. Ed. 2010, 49, 8021.
DOI: 10.1002/anie.201003320)
the elegant SmI2-mediated double cyclization of 11 to
12. This set the stage for the
assembly of (±)-Strychnine (13).
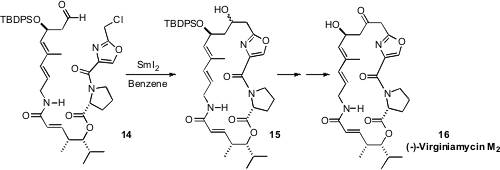
James S. Panek of Boston University used
(Angew. Chem. Int. Ed. 2010, 49, 6165.
DOI: 10.1002/anie.201002220)
the enantiomerically-pure allylic silanes that he has developed to
construct the chloroaldehyde 14. He found that the reductive cyclization to
15 was best carried out with SmI2 in benzene.
SmI2 has the virtue that it is soluble in common organic solvents, so it can
readily be deployed even on a micromole scale. It is also versatile, because its
reducing power can be tuned by the solvent in which it is dissolved.