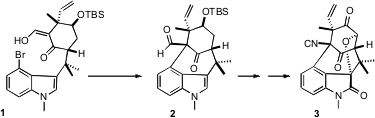
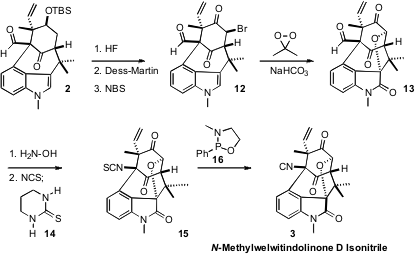
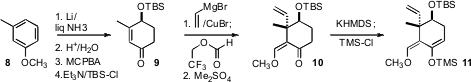The complex polycyclic structure of N-Methylwelwitindolinone D Isonitrile
(3)
was assigned in 1999. The welwitinines show an intriguing range of biological
activity, including reversal of P-glycoprotein-mediated multidrug resistance in
human carcinoma cells. Viresh H. Rawal of the University of Chicago described
(J. Am. PMID:23773119 Chem. Soc. 2011, 133, 5798.
DOI: 10.1021/ja201834u)
the first synthesis of 3, using as a key
step the Pd-catalyzed cyclization of 1 to 2. Price of 3-Bromo-1,1-difluorocyclobutane
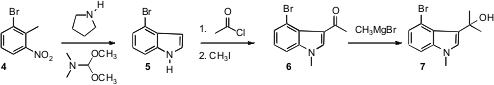
The ketone 1 was assembled by the convergent coupling of 7 with
11. Quinazoline-8-carboxylic acid Chemscene The indole 7 was readily available by Batcho-Leimgruber
cyclization of commercial 4 to 5. The expected 3-acylation
followed by N-methylation delivered the stable ketone 6. The
unstable 7 was prepared as needed.
The anisole 8 was the starting material for the preparation of the alicyclic
diene 11. Although this synthesis was carried out in the racemic series,
enantiomerically-enriched 9 could be prepared by
Shi epoxidation of the β,γ-unsaturated ketone from
Birch reduction followed by hydrolysis.
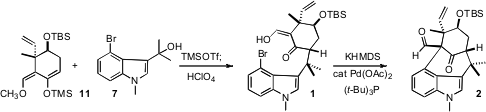
The alcohol 7 was not stable to silica gel chromatography. The mixture of
11 with the crude alcohol 7 was therefore activated by the addition of TMSOTf, then
added via cannula to aqueous HClO4 in THF to deliver the coupled product
1 as a single diastereomer.
The remarkable cyclization of 1 to 2 required extensive screening. Eventually
it was found that a combination of (t-Bu)3P with Pd(OAc)2 as the Pd source
worked well. This concise convergent synthetic strategy makes the welwitinine
core 2 available in gram quantities.
There were two problems to be solved in the conversion of 2 to 3. The first
was the installation of the oxy bridge. Indoles are notoriously sensitive to
over-oxidation. Nevertheless, addition of an acetone solution of dimethyl
dioxirane to the bromo ketone 12 over 24 hours gave clean conversion to
13.
The remaining challenge was the conversion of the aldehyde of 13 to the
isonitrile. Kim had described the inversion of an oxime to the isothiocyanate.
Optimization of this protocol led to the thiourea 14 as the best for this
transformation. Mild desulfurization then delivered N-Methylwelwitindolinone D
Isonitrile (3).