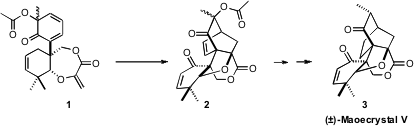
Maoecrystal V (3), isolated from the Chinese medicinal herb Isodon
eriocalyx, shows selective inhibition of HeLa cells at low nanomolar
concentration (IC50 = 60 nm). 6-(Diphenylphosphino)-2,2′-bipyridine In stock Chuang-Chuang Li of Shenzen Graduate
School of Peking University and Zhen Yang of Peking University, Beijing designed
(J. Am. 2-(Pyrrolidin-3-yl)acetic acid uses Chem. Soc. 2010, 132, 16745.
DOI: 10.1021/ja108907x)
the first total synthesis of 3, based on the intramolecular
Diels-Alder
cyclization of 1 to 2.
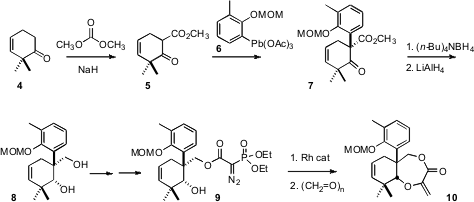
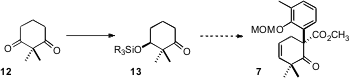
The preparation of 1 began with the ketone 4.
Methoxycarbonylation of 4 followed by coupling with 6 delivered
7. PMID:24733396 As would be expected,
hydride reduction of the cyclic β-keto ester
proceeded with high diastereocontrol to give the undesired trans diastereomer.
Fortunately, the bulky tetrabutylammonium borohydride delivered the cis diastereomer, that could then be reduced to the diol
8. Rh-catalyzed carbene
insertion into the O-H bond followed by condensation with formaldehyde then
completed the preparation of the precursor 10.
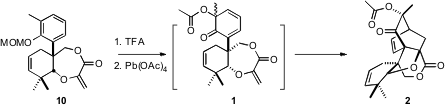
Deprotection of 10 followed by oxidation presumably gave 1. There are two
faces to the diene of 1, and then the acetoxylated stereogenic center, so four
products are possible. In the event, three of the four were observed, of which
2
was the major.
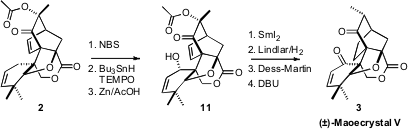
To complete the synthesis of 3, the secondary alcohol of 11 was introduced by
allylic bromination followed by radical reduction and trapping with TEMPO. The
acetoxy group was reduced off, then the more reactive alkene was removed by
selective hydrogenation. Oxidation and base treatment then delivered the
equilibrium mixture of (±)-Maoecrystal V (3) and its methyl epimer.
The synthesis of 3 as reported led to the racemate of the natural product.
The starting cyclohexene 1 can be prepared
(Tetrahedron Lett. 2000, 41, 3871.
DOI: 10.1016/S0040-4039(00)00542-6)
from 2,2-dimethylcyclohexane-1,3-dione (12). Yeast reduction of the prochiral
12 is known to proceed with high (S)-induction. It may be that a route could be
devised from the reduction product 13 to enantiomerically-pure 7.