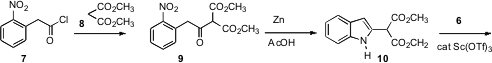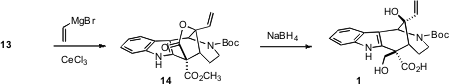(-)-Actinophyllic Acid (3), isolated from Alstonia actinophylla, is a
promising inhibitor of TAFIa/hippicuricase (0.84 µm). Larry E. Overman of UC Irvine envisioned
(J. Am. Chem. 4-Aminomethylbenzylalcohol Price Soc. 2010, 132, 4894.
DOI: 10.1021/ja100178u)
a bold route to 3 based
on the aza-Cope/intramolecular
Mannich reorganization of 1 to 3. (R)-2-amino-1-phenylethan-1-ol Data Sheet
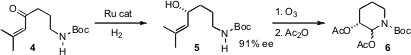
The absolute configuration of 1 and thus of 3 was set by Noyori hydrogenation
of the enone 4.
Ozonolysis followed by acetylation delivered the pyridone
6 as
an inconsequential mixture of diastereomers. PMID:24377291
The ketone 9 was assembled by condensation of dimethyl malonate 8 with the
acid chloride 7. Cyclization then followed directly on reduction of the nitro
group to the amine, to give the crystalline
indole 10. Under Lewis acid
catalysis, 10 coupled smoothly with the diacetate 6, to give 11. Selective
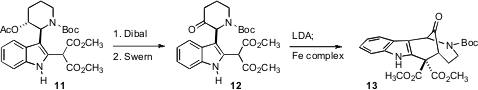
reduction of the acetate was followed by oxidation, leading to 12.
The ketone 12 has only a single acidic stereogenic center. It was not clear
that it could be cyclized without epimerization. A preliminary study with
material resolved by enantioselective chromatography, however, showed that this
in fact worked well. The LDA kinetically deprotonated the ketone away from the
N, at the same time deprotonating the malonate, to give a dianion that underwent
smooth oxidative coupling to 13.
With 13 in hand, it remained to differentiate the two esters derived from the
malonate. This was succinctly accomplished by the addition of vinyl magnesium
bromide. Selective reduction of the spontaneously formed
lactone 14 cleanly
delivered 1.
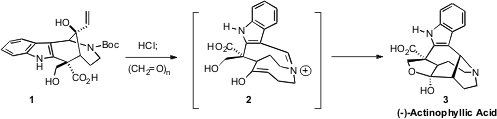
The topological connection between 1 and 3 is not necessarily obvious.
Exposure of 1 to HCl gave the amine hydrochloride. Condensation with
formaldehyde then gave 15, poised for aza-Cope rearrangement to 2. The enol
2,
then, proceeded via intramolecular Mannich condensation directly to (-)-Actinophyllic
Acid (3).