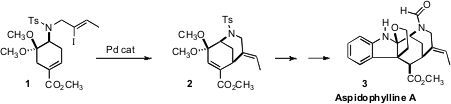
The pentacyclic Apocynaceae alkaloid Aspidophylline A (3) was
shown to reverse drug resistance in resistant KB cells. In developing a strategy
for the assembly of 3, Neil K. PMID:25804060 Garg of UCLA envisioned
(J. Am. Chem. Soc. 2011, 133, 8877.
DOI: 10.1021/ja203227q)
the intramolecular Pd-catalyzed
cyclization of 1 to 2. N-Methylsulfamoyl chloride manufacturer
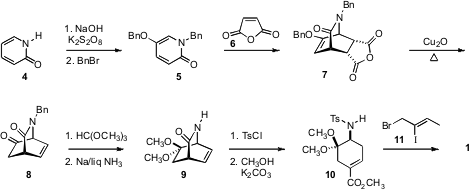
The starting material for the
cyclohexenone derivative 1 was the known
tricyclic anhydride 7. This was readily available in gram quantities by
oxidation of the commercial
pyridone (4). 5-Formylnicotinic acid structure The double decarboxylation to
8 was delicate, but could be effected by iterative small-batch
microwave
heating. Protection of 8 followed by fragmentation and alkylation than
delivered 1.
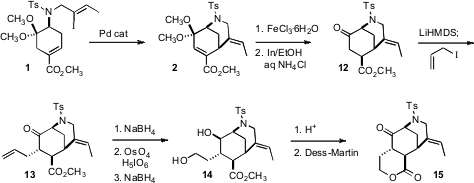
The intramolecular Heck cyclization of 1 indeed proceeded smoothly, to
give the bicyclic diene 2. Deprotection of the ketone revealed a
doubly-activated enone, that could be selectively reduced under modified
dissolving metal conditions to give the keto ester 12. Alkylation of the
lithium enolate with allyl iodide then gave 13, predominantly as the
diastereomer illustrated. Reduction followed by selective Johnson-Lemieux
oxidative cleavage of the terminal alkene then completed the construction of the
diol 14.
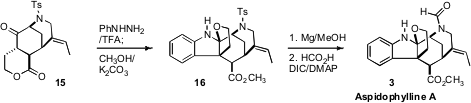
The vision for the final assembly of the alkaloid was to effect interrupted
Fischer indolization of an alkylated
cyclohexanone such as 15. To this
end, several bicyclic ketones were explored, but none was successful. Finally,
attention was turned to the more rigid tricyclic
lactone 15. Happily,
exposure of 15 to phenylhydrazine in the presence of trifluoroacetic acid
led to an intermediate that was not isolated, but rather directly combined with
methanolic K2CO3 to open the lactone, allowing closure of
the tetrahydrofuran ring, to give 16.
Simple arene sulfonamides can be advantageous in synthesis, as they do not
appear as rotameric mixtures in NMR, and are often crystalline. Nevertheless,
they have not commonly been used, because of the perceived difficulty of
deprotection. Sonication of 16 with Mg powder in methanol containing
solid NH4Cl led to smooth desulfonylation. Formylation then completed
the synthesis of Aspidophylline A (3).