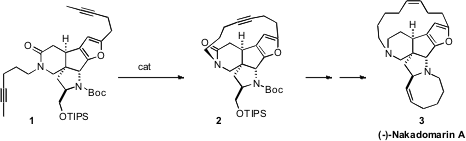
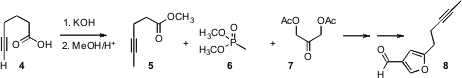The Z alkene of Nakadomarin A (3) suggested to Raymond L. Funk
an approach (Org. Lett. 2010, 12, 4912.
DOI: 10.1021/ol102079z)
based on ring-closing alkyne metathesis. The efficient assembly of 3 he reported
illustrates the power of convergent design in target-directed synthesis. 821785-75-5 structure
A practical limit on applications of alkyne metathesis is the requirement for
internal alkynes, necessitating methyl capping of a terminal alkyne. In an
alternative approach, Professor Funk took advantage of the long-known
(J. 364794-69-4 uses Chem. Soc. PMID:23773119 1954, 3201.
DOI: 10.1039/JR9540003201)
equilibration of a terminal alkyne 4 to
the internal alkyne 5. Homologation of 5 with the phosphonate 6,
followed by condensation with the ketone 7, then delivered the
furan 8.
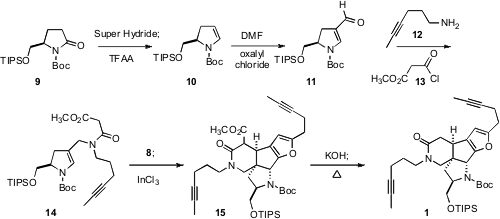
The assembly of the other half of 1 began with the commercial alcohol
derived by reduction of D-pyroglutamic acid. Protection gave 9, that on
hydride addition and dehydration was converted to 10. One-carbon
homologation with the Vilsmeier-Haack reagent proceeded with the expected
regiocontrol. This set the stage for the triply-convergent assembly of 14,
first reductive amination of the aldehyde 11 with 12, then
acylation of the resulting secondary amine with 13. The nucleophilic
14 was condensed with the aldehyde 8 to give an enone (not
illustrated). Exposure of the enone to InCl3 initiated an elegant
cascade cyclization, first of the enamide in a conjugate sense to the enone,
then Friedel-Crafts addition of the resulting N-stabilized carbocation to the
furan, to deliver 15. The pendant silyloxymethyl group exerted the
hoped-for diastereocontrol, allowing the direct construction of the central
tetracycle of 3. Hydrolysis and decarboxylation completed the assembly of
the diyne 1.
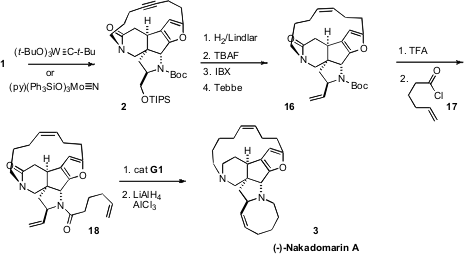
Initially, it was found that exposure of 1 to an Mo catalyst delivered
2 in only modest yield. As an alternative, they employed the technically
more challenging tungsten-based Schrock catalyst. Later, they found that the
recently-developed Fürstner Mo protocol also worked well.
The amide 2 could readily be carried on to the triene 18. With
the first generation Grubbs catalyst (G1), kinetic
ring-closing
metathesis of 18, to complete the assembly of (-)-Nakadomarin (3),
could be effected without jeopardizing the existing Z alkene.