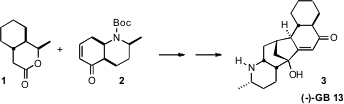An investigation of the activity of the Galbulimima alkaloids, exemplified by
(-)-GB 13, led to the development of a series of potent thrombin receptor
antagonists. Buy138099-40-8 Dawei Ma of the Shanghai Institute of Organic Chemistry devised
(Angew. Chem. Int. PMID:24140575 Ed. 2010, 49, 5887.
DOI: 10.1002/anie.201002299)
a concise route to 3 based on the coupling of the chirons 1 and 2. 725728-43-8 supplier
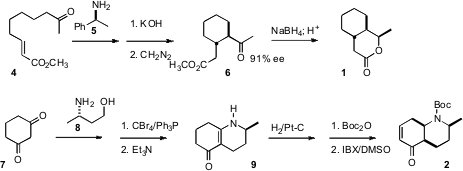
The starting point for the preparation of 1 was the unsaturated ester
4. Cyclization using the chiral enamine protocol developed by d’Angelo
delivered the keto ester 6. Reduction with
NaBH4 proceeded
with substantial diastereocontrol to give an intermediate alcohol, that cyclized
under acidic conditions to the
lactone 1.
The preparation of 2 began with dihydroresorcinol 7.
Condensation with the enantiomerically-pure amine 8 gave the enamine,
that was converted to the bromide and cyclized to 9. Hydrogenation with
substantial facial control set the ring fusion. Oxidation with 2-iodoxybenzoic
acid (IBX) in DMSO introduced unsaturation with high regioselectivity, to give
2.
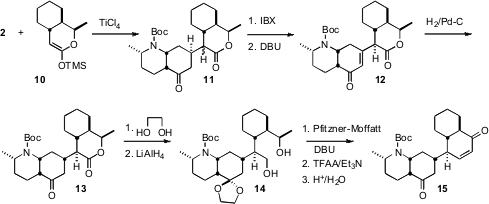
The ketene silyl acetal 10 derived from the lactone 1 added
under Mukaiyama conditions across the open face of 2, to give the adduct
11. The same
IBX oxidation protocol was used to introduce unsaturation,
and the product was equilibrated to give 12. Hydrogenation, again across
the open face of the bicyclic enone, then set the last stereogenic center of
13.
To construct the
cyclohexenone 15, it was necessary to oxidize the
diol 14 to the keto aldehyde. Others had found that the Swern
modification of the Pfitzner-Moffatt oxidation worked well in such cases,
minimizing competing lactone formation. Even more useful was the Boger protocol,
that returned to the original Pfitzner-Moffatt conditions, activating DMSO with
TFAA. Use of DBU in place of the more typical Et3N then cleanly
delivered the aldol product, that was dehydrated and deprotected to give the
enone 15.
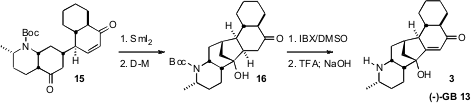
The final ring closure was effected by reduction of the enone with
SmI2.
The initially-formed 16 was partially reduced to the diol under the
reaction conditions, necessitating re-oxidation with the
Dess-Martin reagent.
The introduction of unsaturation with the Nicolaou IBX protocol was again
successful, even in this more complex and fragile system. Deprotection then
completed the synthesis of 3.