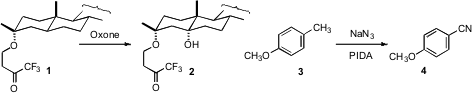
Masayuki Inoue of the University of Tokyo designed(Org. Lett. 2009, 11, 3630.DOI: 10.1021/ol901367m)a linker that specifically directed C-H hydroxylation, as illustrated by oxidationof 1 to 2. Phil S. Baran of Scripps/La Jolla detailed(Nature 2009, 459, 824,DOI: 10.1038/nature08043;Angew. Chem. Int. Ed. 2009, 48, 9705,DOI: 10.1002/anie.200904474)some of the factors that direct reactivity in intermolecular C-H hydroxylation.Ning Jiao of Peking University devised(Angew. Chem. Int. Buy1190321-59-5 Ed. 2009, 48, 7094.DOI: 10.1002/anie.200903838)a protocol for the direct oxidation of a methylated aromatic 3 to the nitrile 4. PMID:34235739 (S)-BINAPINE uses
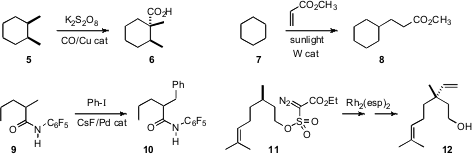
Armando J. L. Pombeiro of TU Lisbon developed(Adv. Synth. Catal. 2009, 351, 2936.DOI: 10.1002/adsc.200900537)a procedure for C-H carboxylation, converting 5 into 6.Maurizio Fagnoni of the University of Pavia showed(Chem. Commun. 2009, 7351.DOI: 10.1039/B917732A)that sunlight was sufficient to promote the addition of cyclohexane 7to methyl acrylate to give 8. Jin-Quan Yu, also of Scripps/La Jolla, established(J. Am. Chem. Soc. 2009, 131, 9886.DOI: 10.1021/ja903573p)conditions for the selective Pd-mediated coupling of 9 to iodobenzeneto give 10. Alexei V. Novikov of the University of North Dakota demonstrated(Tetrahedron Lett. 2009, 50, 6963,DOI: 10.1016/j.tetlet.2009.09.147;Heterocycles 2009, 78, 2531,DOI: 10.3987/COM-09-11788)that both diazo sulfonates such 11 and the related diazo sulfones cyclizedsmoothly under Rh catalysis to give the six-membered ring products. In the course ofa synthesis of the Psoralea corylifolia-derived Bakuchiol, the adduct from thecyclization of 11 was converted into the vinylated product 12.
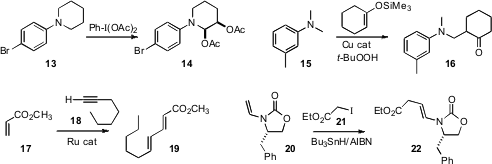
C-H bonds can also be activated electronically by proximal functional groups. Yong-Min Liang of Lanzhou University observed(J. Org. Chem. 2009, 74, 7464.DOI: 10.1021/jo901583r)that an N-aryl cyclic amine 13 could be oxidized to the syn diacetoxylatedproduct 14. Note that the α-acetoxy group of 14 is activated for ionizationand further bond formation. Yuhong Zhang of Zhejiang University, Hangzhou found(Org. Lett. 2009, 11, 3730.DOI: 10.1021/ol901347t)that the activated intermediate from the oxidation of 15 coupled with a silylenol ether to deliver the coupled product 16.
Bernd Plietker of the Universität Stuttgart devised(Angew. Chem. Int. Ed. 2009, 48, 5752.DOI: 10.1002/anie.200901928)a Ru catalyst for the coupling of an alkyne 18 to an α,β-unsaturated ester 17 to give the diene 19. Both disubstituted alkynes and morecomplex α,β-unsaturated esters participated as well. Gregory K. Friestadof the University of Iowa observed(Org. Lett. 2009, 11, 819.DOI: 10.1021/ol8028077)that an N-vinyl amide 20 could be homologated to the ester 22.
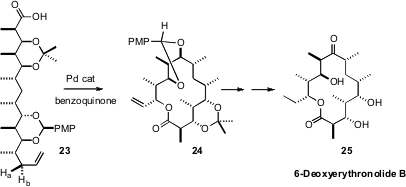
Allylic C-H bonds can also be selectively oxidized. In an elegant demonstrationof C-H functionalization, M. Christina White of the University of Illinois showed(Nat. Chem. 2009, 1, 547.DOI: 10.1038/nchem.351)that 23 could be cyclized to a single diastereomer of the macrolide 24.She carried 24 on to 6-Deoxyerythronolide (25), the aglycone precursor ofthe erythromycin antibiotics.