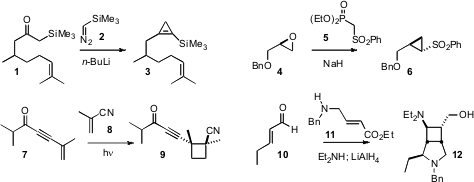
Daesung Lee of the University of Illinois, Chicago, taking advantage of the
facile insertion of an alkylidene carbene into a C-Si bond, established
(J. Am. PMID:34856019 Chem. Soc. 2010, 132, 6640.
DOI: 10.1021/ja101998w) a general
method for the conversion of an α-silyl ketone 1 into the silyl
cyclopropene 3. Christopher D. Ammonium iron(III) citrate Purity Bray of Queen Mary University showed
(J. Org. Chem. 2010, 75, 4652.
DOI: 10.1021/jo100844g)
that the sulfonyl phosphonate 5 converted the enantiomerically-pure epoxide 4
into the cyclopropane 6. Paul Margaretha of the University of Hamburg observed
(Org. Lett. 2010, 12, 728.
DOI: 10.1021/ol902827s)
smooth photochemical combination of 7 and 8 to give 9 with
high diastereocontrol. Tõnis Kanger of the Tallinn University of Technology devised
(Org. Lett. 2010, 12, 2230.
DOI: 10.1021/ol1005714)
the three-component coupling of 10, 11, and diethyl
amine to give, after reduction, the highly-substituted cyclobutane 12.
Min Shi of the Shanghai Institute of Organic Chemistry uncovered
(J. 5-Bromo-1,3-dihydroisobenzofuran Order Org. Chem. 2010, 75, 902.
DOI: 10.1021/jo902512q)
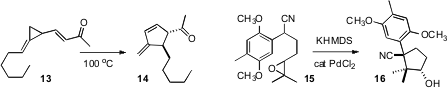
an interesting new thermal rearrangement, the conversion of 13 to 14.
José G. Ávila-Zárraga of the Universidad Nacional Autónoma de México applied
(Tetrahedron Lett. 2010, 51, 2232.
DOI: 10.1016/j.tetlet.2010.02.072)
Pd catalysis to the cyclization of the epoxy nitrile 15, redirecting the
reaction from the expected
cyclobutane to the cyclopentanol 16. Ullrich Jahn
of the Academy of Sciences of the Czech Republic effected
(J. Org. Chem. 2010, 75, 4480.
DOI: 10.1021/jo1006569)
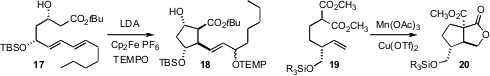
the oxidative radical cyclization of 17 to 18. Initial
deprotonation of the substrate with t-BuMgCl switched the product to the
trans diastereomer. Jonathan W. Burton of the University of Oxford employed
(Org. Lett. 2010, 12, 2738.
DOI: 10.1021/ol100794k)
a related oxidative cyclization for the diastereoselective conversion of 19 to 20.
E. J. Corey of Harvard University reported
(Org. Lett. 2010, 12, 300.
DOI: 10.1021/ol902643w)
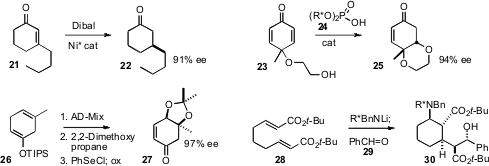
a new ligand for the enantioselective Ni-mediated reduction of 21 to 22.
Shu-Li You, also of the Shanghai Institute of Organic Chemistry, established
(J. Am. Chem. Soc. 2010, 132, 4056.
DOI: 10.1021/ja100207s)
that the alcohol 23, readily prepared by oxidation of p-cresol, could be
cyclized to the crystalline 25 in high ee. Seth B. Herzon of Yale University prepared
(J. Am. Chem. Soc. 2010, 132, 2540.
DOI: 10.1021/ja910769j)
the crystalline enone 27, also in high ee, from the
Birch reduction product 27.
For related approaches to the enantioselective oxidation of benzene derivatives, see
Chem. Commun. 2010, 701, DOI: 10.1039/B920884D and
Org. Lett. 2010, 12, 2642, DOI: 10.1021/ol100840n.
Manabu Node of Kyoto Pharmaceutical University optimized
(J. Org. Chem. 2010, 75, 4201.
DOI: 10.1021/jo1004586)
an enantiomerically-pure amine for the cyclization of 28 to the
highly-substituted
cyclohexane 30. The chiral amine was easily removed and
recycled by oxidation to the corresponding aldehyde.
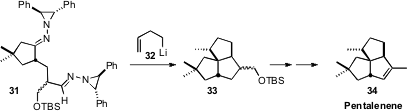
Sunggak Kim, now at Nanyang Technical University, developed
(Synlett 2010, 1511.
DOI: 10.1055/s-0029-1219933)
the anionic chemistry of imines derived from N-amino diphenyl aziridine. He showed that addition of
3-butenyl lithium to 31 initiated a cascade cyclization. The tricyclic product
33 was readily converted to the sesquiterpene Pentalenene 34.