Synthesis of (+)-Bourgeanic Acid
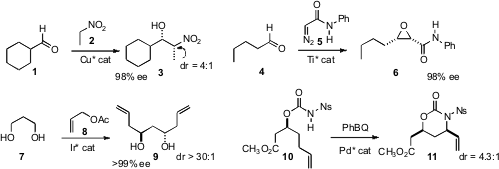
Kyungsoo Oh of Indiana University Purdue University Indianapolis devised
(Org. (S)-(-)-3-Butyn-2-ol Order Lett. 2009, 11, 5682.
DOI: 10.1021/ol902380z)
a new ligand that with Cu delivered predominantly one diastereomer
of the Henry adduct 3, and with Zn delivered the other.
Liu-Zhu Gong of the University of Science and Technology of China reported
(Angew. Chem. Int. Ed. 2009, 48, 6503.
DOI: 10.1002/anie.200903061)
the Darzens condensation of the diazoacetamide 5 with a variety of aldehydes
to give the corresponding epoxy amides with high diastereo- and enantiocontrol.
Michael J. Krische of the University of Texas, Austin applied
(Org. Lett. Potassium trichloroammineplatinate(II) uses 2009, 11, 3108,
DOI: 10.1021/ol901096d; 3112,
DOI: 10.1021/ol901136w)
his asymmetric allylation to a variety of
primary diols including 7, leading to the homologated product 9.
M. Christina White of the University of Illinois showed
(J. PMID:24065671 Am. Chem Soc. 2009, 131, 11707.
DOI: 10.1021/ja9054959)
that Pd-mediated oxidative amination of carbamate 10 delivered the protected
1,3-amino alcohol 11 with high diastereocontrol.
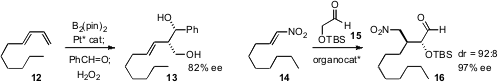
James P. Morken of Boston College devised
(J. Am. Chem Soc. 2009, 131, 9134.
DOI: 10.1021/ja809610h)
a Pt catalyst for the asymmetric bis-boration of dienes. The
allyl borane prepared from 12 added with high stereocontrol to
benzaldehyde, to give, after oxidation, the diol 13. Carlos F. Barba III
of Scripps La Jolla optimized
(Angew. Chem. Int. Ed. 2009, 48, 9848.
DOI: 10.1002/anie.200905313)
an organocatalyst for the enantioselective
conjugate addition of an alkoxy
aldehyde 15 to a nitroalkene. Do Hyun Ryu of Sungkyunkwan University found
(Chem. Commun. 2009, 5460.
DOI: 10.1039/b910321j)
that an organocatalyst could also mediate the
dipolar cycloaddition of a diazo ester
18 to an unsaturated aldehyde, to give 19 with high diastereo- and
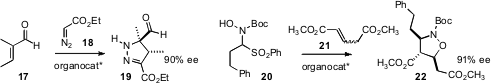
enantiocontrol. Francesco Fini and Luca Bernardi of the University of Bologna developed
(J. Am. Chem Soc. 2009, 131, 9614.
DOI: 10.1021/ja902458m)
an organocatalyst that effected enantioselective dipolar cycloaddition of the nitrone
derived from 20 to the unsaturated ester 21.
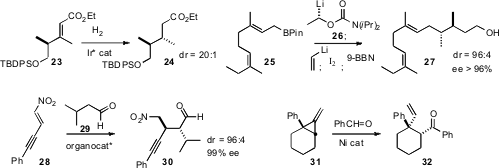
Kevin Burgess of Texas A&M optimized
(J. Am. Chem Soc. 2009, 131, 13236.
DOI: 10.1021/ja905458n)
an Ir catalyst for the enantioselective hydrogenation of trisubstituted
alkenes such as 23. In the course of a synthesis of (+)-Faranal,
Varinder K. Aggarwal of the University of Bristol described
(Angew. Chem. Int. Ed. 2009, 48, 6317.
DOI: 10.1002/anie.200901194)
a one-pot procedure for the conversion of the
allyl boronate 25 into 27.
Bernhard Breit of Albert-Ludwigs-Universität, Freiburg reported
(Org. Lett. 2009, 11, 4668.
DOI: 10.1021/ol901944b)
related work. Norbert Krause of the Technische Universität
Dortmund and Alexandre Alexakis of the Université de Genève extended
(Angew. Chem. Int. Ed. 2009, 48, 8923.
DOI: 10.1002/anie.200903905)
organocatalyzed aldehyde conjugate addition to alkynyl nitroalkenes such as
28. Toshimichi Ohmura and Michinori Suginome of Kyoto University observed
(J. Am. Chem Soc. 2009, 131, 11298.
DOI: 10.1021/ja9046894)
that the Ni-catalyzed opening of the methylidene cyclopropane 31
proceeded with high regioselectivity. Several routes to enantiomerically-pure
methylidene cyclopropanes have been reported.
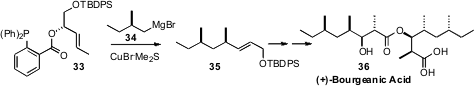
For fragments such as 34 to be used in synthesis, the convergent
coupling must proceed with high efficiency. Professor Breit developed
(Org. Lett. 2009, 11, 3286.
DOI: 10.1021/ol9011635)
the o-diphenylphosphinobenzoate 33 for this purpose.
In the course of a synthesis of the dimeric natural
product (+)-Bourgeanic Acid (36), 33 was coupled with just 1.5
equivalents of the Grignard reagent 34 to give 35 in 83% yield.