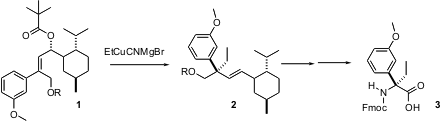
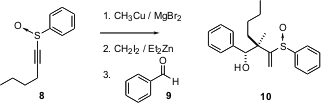The direct enantioselective synthesis of quaternary centers is one of the enduring challenges of organic synthesis. Claude Spino of the Université de Sherbrooke reports (J. Am. Chem. Buy1801273-41-5 1255352-25-0 Price Soc. 2003, 125, 12106.DOI: 10.1021/ja037078a)that organocuprate addition to the secondary pivalate 1 proceeds with outstanding diastereocontrol, to give 2 with an enantiodefined alkylated quaternary center. PMID:24360118 The homoallylic alcohols so prepared were efficiently converted into the corresponding enantiomerically-pure protected α,α-dialklyated α-amino acids, such as3.
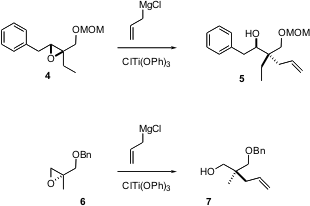
Tetsuaki Tanaka of Osaka University has reported (Tetrahedron Lett. 2004,45, 75.DOI: 10.1016/j.tetlet.2003.10.112)what appears to be a general route to alkylated quaternary centers, based on the Ti-mediated addition of allylmagnesium chloride to the Sharpless-derived epoxy ether4. Remarkably, the conversion of 6 to 7 works equally well.
Enantiomerically-pure sulfoxides are readily available. Ilan Marek of Technion-Israel Institute of Technology reports (J. Am. Chem. Soc. 2003, 125, 11776.DOI: 10.1021/ja036872t)that alkyne-derived sulfoxides such as 8 can be used to direct the addition of an allylic organometallic, prepared in situ, to an aldehyde9. Both the secondary alcohol, from the aldehyde, and the adjacent quaternary center of 10 are formed with >99%stereocontrol.