Lycoposerramine-C
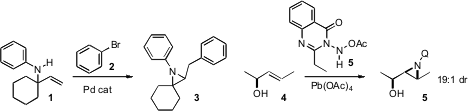
Hideki Yorimitsu and Koichiro Ochima of Kyoto University extended
(Angew. Chem. Int. Ed. 2009, 48, 7224.
DOI: 10.1002/anie.200903178)
Pd-catalyzed intramolecular carboamidation to the construction of aziridines such
as 3. Hamdullah Kilic of Ataturk University showed
(J. Org. Chem. 2009, 74, 9452.
DOI: 10.1021/jo902092s)
that aziridination of an allylic alcohol 4 could proceed with
substantial diastereocontrol. 6-Amino-3-bromopicolinonitrile Chemscene
Makoto Oba of Tokai University established
(Tetrahedron Lett. PMID:24025603 2009, 50, 5053.
DOI: 10.1016/j.tetlet.2009.06.096)
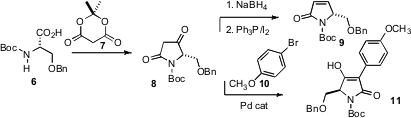
a route from a serine derivative 6 to the pyroglutamate
8, and developed a protocol for the conversion of 8 to 9.
David Tanner of the Technical University of Denmark found
(J. Org. Chem. 2009, 74, 5032.
DOI: 10.1021/jo900799y)
that tetramic acids such as 8 could also be efficiently α-arylated. 2439223-60-4 Chemscene
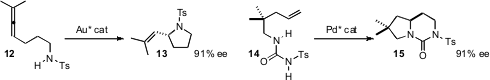
Koichi Mikami of the Tokyo Institute of Technology devised
(Angew. Chem. Int. Ed. 2009, 48, 6073.
DOI: 10.1002/anie.200902084)
a gold catalyst for the enantioselective cyclization of 12
to 13. Hiroaki Sasai of Osaka University effected
(J. Org. Chem. 2009, 74, 9274.
DOI: 10.1021/jo901778a)
double intramolecular amination, converting 14 into 15 in high ee.
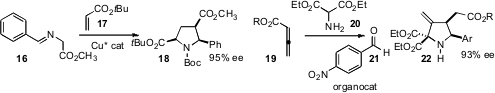
Kyungsoo Oh of IUPUI Indianapolis observed
(Angew. Chem. Int. Ed. 2009, 48, 7420.
DOI: 10.1002/anie.200903479)
that using the same ligand, Cu catalysis gave one enantiomer of 18, and Ag catalysis gave
the opposite enantiomer. Liu-Zhu Gong of the University of Science and Technology, Hefei devised
(Org. Lett. 2009, 11, 4946.
DOI: 10.1021/ol9020964)
a chiral Brønsted acid that mediated the enantioselective condensation of
19, 20, and 21 to give 22.
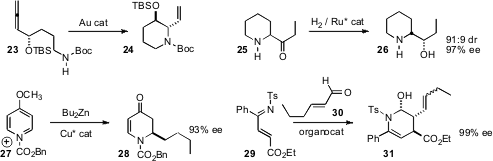
Roderick W. Bates of Nanyang Technological University found
(Org. Lett. 2009, 11, 3706.
DOI: 10.1021/ol901094h)
that a gold catalyst mediated the cyclization of 23 to 24 with
high diastereocontrol. Qi-Lin Zhou of Nankai University effected
(Org. Lett. 2009, 11, 4994.
DOI: 10.1021/ol901605a)
the enantioselective reduction of racemic 25 under epimerizing
conditions, leading to 26 with high stereocontrol. Adriaan J. Minnaard and Ben
L. Feringa of the University of Groningen established
(Angew. Chem. Int. Ed. 2009, 48, 9339.
DOI: 10.1002/anie.200904981)
conditions for the enantioselective addition of a diakyl zinc to
the activated pyridine 27, to give 28 in high ee. Ying-Chun Chen of the West
China School of Pharmacy showed
(Angew. Chem. Int. Ed. 2009, 48, 5474.
DOI: 10.1002/anie.200902216)
that an organocatalyst could mediate the condensation of 29 with 30 to give
31 in high ee.
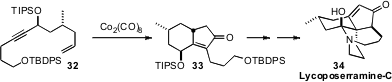
Hiromitsu Takayama of Chiba University isolated
(Tetrahedron Lett. 2002, 43, 8307.
DOI: 10.1016/S0040-4039(02)02026-9)
Lycoposerramine-C (34) from Lycopodium serratum. Intrigued by preliminary
studies of the biological activity but lacking material, he developed
(Org. Lett. 2009, 11, 5554.
DOI: 10.1021/ol902437t)
a total synthesis, the key step of which was the
intramolecular Pauson-Khand cyclization of 32 to 33.