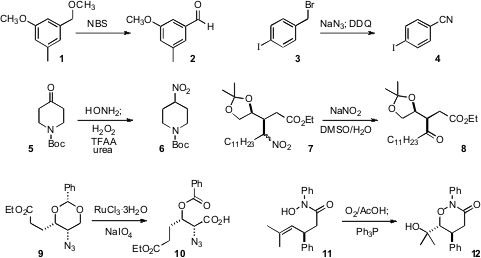
Mark Cushman of Purdue University found
(J. Org. Chem. 2010, 75, 3507.
DOI: 10.1021/jo1004313)
that a benzylic methyl ether 1 could be converted to the
aldehyde 2 by N-bromosuccinimide. Two equivalents of
NBS gave the
methyl ester. Ning Jiao of Peking University used
(Org. 1,2,3-Triaminoguanidine;hydrochloride Chemscene Lett. 2010, 12, 2888.
DOI: 10.1021/ol101094u)
NaN3 followed by
DDQ to oxidize a benzylic halide
3 to the nitrile 4. Hugues Miel of Almac Sciences oxidized
(Tetrahedron Lett. 2010, 51, 3216.
DOI: 10.1016/j.tetlet.2010.04.046)
the ketone 5 to the nitro derivative 6. The
oxidative conversion
of the nitro compound 7 to the ketone 8 described
(Tetrahedron Lett. 2009, 50, 6389.
DOI: 10.1016/j.tetlet.2009.08.087)
by Vera L. Patrocinio Pereira of the Universidade Federal do Rio de
Janeiro proceeded without epimerization.
Sundarababu Baskaran of the Indian Institute of Technology Madras established
(Angew. PMID:23618405 Chem. Int. (3-Chloronaphthalen-2-yl)boronic acid supplier Ed. 2010, 49, 804.
DOI: 10.1002/anie.200905952)
that oxidative cleavage of the benzylidene acetal 9 delivered 10 with high
regioselectivity. The intramolecular alkene
dihydroxylation of 11 originated
(Angew. Chem. Int. Ed. 2010, 49, 4491.
DOI: 10.1002/anie.201000843)
by Erik J. Alexanian of the University of North Carolina gave 12 with high diastereocontrol.
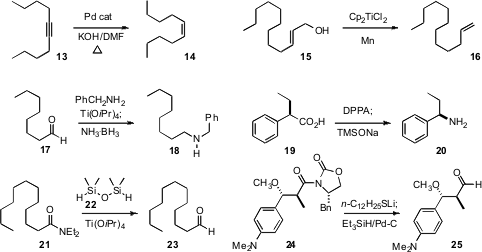
Ruimao Hua of Tsinghua University took advantage
(J. Org. Chem. 2010, 75, 2966.
DOI: 10.1021/jo100247a)
of the H-donor properties of DMF to develop an efficient
semireduction of the alkyne
13 to the alkene 14. Alejandro F. Barrero of the University of Granada developed
(J. Am. Chem. Soc. 2010, 132, 254.
DOI: 10.1021/ja906083c)
Ti (III) conditions for the reduction of the allylic alcohol 15 to
the terminal alkene 16. Isolated alkenes were stable to these conditions.
P. Veeraraghavan Ramachandran, also of Purdue University, effected
(Tetrahedron Lett. 2010, 51, 3167.
DOI: 10.1016/j.tetlet.2010.04.014)
reductive amination of 17 to 18 using the now readily available
NH3-BH3. Bin Ma and Wen-Cherng Lee of BiogenIdec developed
(Tetrahedron Lett. 2010, 51, 385.
DOI: 10.1016/j.tetlet.2009.11.038)
a simple protocol for the conversion of an acid 19 to the free amine 20.
Marc Lemaire of Université Lyons 1 established
(Tetrahedron Lett. 2010, 51, 2092.
DOI: 10.1016/j.tetlet.2010.02.008)
that the silane 22 reduced primary, secondary and tertiary amides to
the aldehydes. Paul Helquist of the University of Notre Dame displaced
(J. Org. Chem. 2010, 75, 2061.
DOI: 10.1021/jo902422y)
the acyl oxazolidineone of 24 with a thiolate, to give
a thioester that was
readily reduced to the aldehyde 25, again without epimerization.
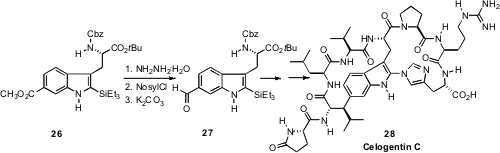
Reduction of the ester 26 provided the first supply of the aldehyde
27, a key early intermediate in the synthesis of Celogentin C (28) described
(J. Am. Chem. Soc. 2010, 132, 1159.
DOI: 10.1021/ja909870g)
by Steven L. Castle of Brigham Young University. Following the Braslau modification of the
McFadyen-Stevens conditions, selective reaction of the methyl ester of 26
with hydrazine gave the hydrazide, that was sulfonylated with
2-nitrobenzenesulfonyl chloride. Exposure of the nosyl acyl hydrazide to mild
base liberated the aldehyde 27.