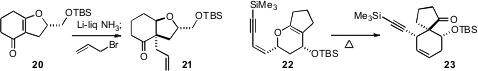Tehshik P. Yoon of the University of Wisconsin uncovered
(J. Am. Chem. Soc. 2009, 131, 14604.
DOI: 10.1021/ja903732v)
conditions for the crossed photodimerization of
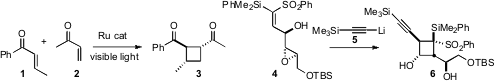
acyclic enones. 1019158-02-1 web Minoru Isobe of Nagoya University extended
(Synlett 2009, 1157.
DOI: 10.1055/s-0028-1088108)
conjugate addition-intramolecular epoxide opening to substrates
such as 4, leading to the
cyclobutane 6 with high diastereocontrol. PMID:23937941
In the course of a total synthesis of (+)-Brefeldin A, Jinsung Tae of Yonsei
University established
(Synlett 2009, 1303.
DOI: 10.1055/s-0029-1216723)
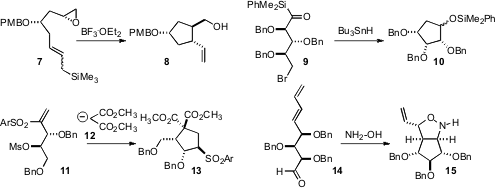
conditions for the trans-selective cyclization of 7 to 8.
Cyclization with TiCl4 gave the alternative cis diastereomer.
Several methods have been put forward for the conversion of carbohydrate
derivatives to carbocycles. Yeun-Mi Tsai of the National Taiwan University found
(Tetrahedron Lett. 2009, 50, 3805.
DOI: 10.1016/j.tetlet.2009.04.032)
that acyl silanes such as 9 cyclized efficiently under free radical
conditions, leading to the silyl ether 10. Tanmaya Pathak of the
Indian Institute of Technology, Kharagpur developed
(Eur. J. Org. Chem. Benzo[d]thiazole-4-carboxylic acid Purity 2009, 872.
DOI: 10.1002/ejoc.200900957)
the tandem conjugate addition-intramolecular alkylation conversion of 11 to
13. Slawomir Jarosz of the Polish Academy of Sciences, Warsawza observed
(Heterocycles 2009, 80, 1303.
DOI: 10.3987/COM-09-S(S)120)
that the oxime derived from 14 cyclized to
15. The cyclization was accelerated by high pressure.
Cyclohexanes can also be prepared from carbohydrates. Tony K. M. Shing of the
Chinese University of Hong Kong showed
(Org. Lett. 2009, 11, 5070.
DOI: 10.1021/ol902071e)
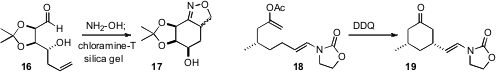
that the nitrile oxide derived from 16 cyclized to 17, that he carried
on to (-)-Gabosine O. John K. Gallos of the Aristotle University of Thessaloniki described
(Tetrahedron Lett. 2009, 50, 6916.
DOI: 10.1016/j.tetlet.2009.09.154)
related work. Paul E. Floreancig of the University of Pittsburgh devised
(Org. Lett. 2009, 11, 3152.
DOI: 10.1021/ol901188q)
conditions for the oxidative cyclization of 18 to 19. Ring closure
proceeded with high equatorial selectivity. Kou Hiroya of Tohoku University found
(J. Org. Chem. 2009, 74, 6623.
DOI: 10.1021/jo901094x)
that the single oxygenated stereogenic center of 20
directed the dissolving metal reduction-enolate trapping, leading to 21.
Similarly, Susumu Kobayashi of the Tokyo University of Science showed
(Synlett 2009, 1605.
DOI: 10.1055/s-0029-1217339)
that the oxygenated stereogenic centers of 22 set the alkylated centers of 23.
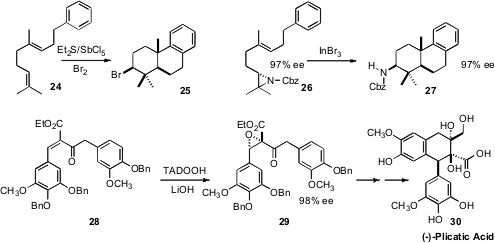
Many marine organisms are able to carry out brominative and chlorinative
polyolefin cyclizations. Scott A. Snyder of Columbia University established
(Angew. Chem. Int. Ed. 2009, 48, 7899.
DOI: 10.1002/anie.200903834)
that the reagent prepared by adding Br2 to a mixture of Et2S
and SbCl5 effected the cyclization of 24 to 25. In a related
development, Teck-Peng Loh of Nanyang Technological University demonstrated
(Chem. Commun. 2009, 3738.
DOI: 10.1039/B903696B)
that the aziridine 26 cyclized efficiently to 27.
Li Deng of Brandeis University described
(J. Am. Chem. Soc. 2009, 131, 10384.
DOI: 10.1021/ja9039407)
the remarkable enantioselective epoxidation of 28 to 29.
Intramolecular Friedel-Crafts cyclization of 29 led on to (-)-Plicatic Acid (30).