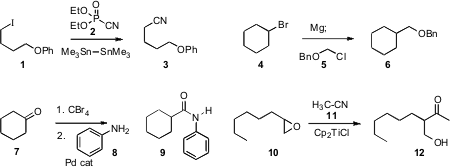
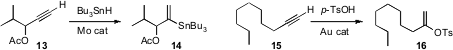Sunggak Kim of KAIST reported(Synlett 2009, 81.DOI: 10.1055/s-0028-1087385)an improved protocol for the one-carbon free radical homologation of an iodide such as1 to the nitrile. Primary, secondary and tertiary iodides work well. We described(Tetrahedron Lett. 2009, 50, 2462.DOI: 10.1016/j.tetlet.2009.03.010)a procedure for the one-carbon homologation of a halide 4 directlyto the benzyl ether 6. Bin Xu of Shanghai University showed(Chem. 4-Bromo-3,5-dimethylphenylboronic acid In stock Commun. 2009, 3246.DOI: 10.1039/B904991F)that conversion of a ketone 8 to the 1,1-dibromoalkene set the stage for the net one-carbon homologation to the amide 9.
A. Fernández-Mateos of the Universidad de Salamanca uncovered(J. Org. Chem. Formula of 85559-46-2 2009, 74, 3913.DOI: 10.1021/jo900479v)a powerful new branching reaction, condensing the more substituted center of anepoxide 10 with a nitrile 11, to deliver the adduct 12. Usefuldiastereocontrol was observed with cyclic epoxides.
Uli Kazmaier of the Universität des Saarlandes optimized(Adv. Synthy. PMID:25105126 Catal. 2009, 351, 1395.DOI: 10.1002/adsc.200900093)a Mo catalyst for the hydrostannation of a terminal alkene 13to the branched product 14. Dong-Mei Cui of the Zhejiang Universityof Technology and Chen Zhang of Zhejiang University, both in Hangzhou, developed(Chem. Commun. 2009, 1577.DOI: 10.1039/B818863G)a complementary procedure, converting the terminal alkene 15 into the branched alkenyl tosylate 16. The Wittig reaction is notorious for racemizing sensitive aldehydes. Hélène Lebel of the Université de Montréal demonstrated(Org. Lett. 2009, 11, 41.DOI: 10.1021/ol802299d)a simple one-pot protocol for sequential oxidation and homologation of 17 that preserved the integrity of the adjacent stereogenic center.
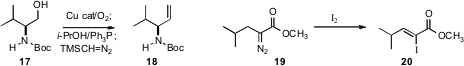
The stereocontrolled construction of trisubstituted alkenes is still a major issuein organic synthesis. Giancarlo Verardo of the University of Udine established(J. Phys. Org. Chem. 2009, 22, 24.DOI: 10.1002/poc.1421)that the α-diazo ester 19, readily prepared directly from thesimple ester, was converted by I2 to the alkene 20 with high geometric control.
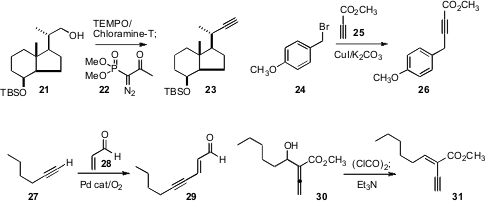
Condensation with the Ohira reagent 22 is often the method of choice for converting an aldehyde into the homologated alkyne. Hubert Maehr and Milan Uskokovic of Bioxell and Carl P. Schaffner of the Waksman Institute described (Syn. Commun. 2009, 39, 299.DOI: 10.1080/00397910802372566)an optimized, scalable procedure for the in situ preparation of 22and the conversion of 21 to 23. Note, again, that the sensitive stereogenic center adjacent to the intermediate aldehyde was not epimerized under the reaction conditions.
Jeremy E. Wulff of the University of Victoria devised(J. Org. Chem. 2009, 74, 3997.DOI: 10.1021/jo900444x)a method for coupling an alkyne with a benzylic halide. Even sensitive, electron-poor alkynes such as 25 worked well.Kyun Woon Jung of the University of Southern California developed(Tetrahedron Lett. 2009, 50, 2370.DOI: 10.1016/j.tetlet.2009.02.217)a catalyst system for the oxidative coupling of a terminal alkyne27 with acrolein 28 to give 29. The oxidative coupling worked well with acrylates also.
Combining alkyne synthesis with the stereocontrolled construction of a trisubstituted alkene, Phil Ho Lee of Kangwon National University(Org. Lett. 2009, 11, 1445.DOI: 10.1021/ol9001703)and Shenming Ma of the Shanghai Institute of Organic Chemistry(Org. Lett. 2009, 11, 2169.DOI: 10.1021/ol9004273)showed that the allenyl alcohol 30 could be dehydrated to the enyne31. The adduct 30 was readily prepared from the corresponding aldehyde.