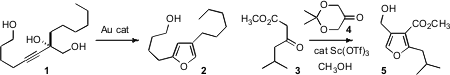
Simultaneously, Aaran Aponick of the University of Florida
(Org. Lett. 2009, 11, 4624.
DOI: 10.1021/ol901901m)
and Shuji Akai of the University of Shizuoka
(Org. Lett. 2009, 11, 5002.
DOI: 10.1021/ol901942t)
reported the Au-mediate conversion of a propargylic diol such as 1 to the
furan 2.
Pyrroles can also be prepared using the same protocol. Jason K. Sello of Brown University developed
(Org. MC-Val-Cit-PAB Formula Lett. 2009, 11, 2984.
DOI: 10.1021/ol9009893)
the direct aldol condensation of an acetoacetate 3 with the protected 1,3-dihydroxy acetone
4 to give 5, the methyl ester of a methylenomycin furan (MMF) bacterial signaling
molecule from Streptomyces coelicolor.
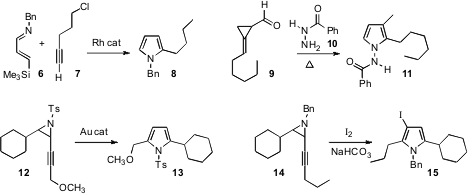
Nobuharu Iwasawa of the Tokyo Institute of Technology demonstrated
(Angew. Chem. Int. PMID:24834360 Ed. 2009, 48, 8318.
DOI: 10.1002/anie.200904402)
that the imine 6 was sufficiently nucleophilic to react with the Rh vinylidene derived from the
alkyne 7, leading to the pyrrole 8. Min Shi of the Shanghai Institute of Organic Chemistry extended
(J. Org. Chem. 4-(6-Bromopyridin-3-yl)morpholine Purity 2009, 74, 5983.
DOI: 10.1021/jo900730t)
the reactivity of methylene
cyclopropanes to the condensation of the aldehyde 9 with an acyl hydrazide, to give the pyrrole 11.
Xue-Long Hou, also of the Shanghai Institute of Organic Chemistry, described
(Tetrahedron Lett. 2009, 50, 6944.
DOI: 10.1016/j.tetlet.2009.05.091)
the Au-mediated reorganization of the alkynyl aziridine 12
to the pyrrole 13. Masahiro Yoshida of the University of Tokushima carried out
(Tetrahedron Lett. 2009, 50, 6268.
DOI: 10.1016/j.tetlet.2009.09.004)
a similar rearrangement under oxidative conditions, to give the iodinated pyrrole 15.
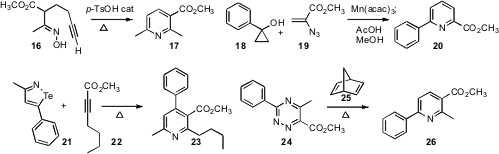
André M. Beauchemin of the University of Ottawa showed
(Angew. Chem. Int. Ed. 2009, 48, 8325.
DOI: 10.1002/anie.200903922)
that under acid catalysis, the oxime 16 cyclized to the
pyridine 17.
Shunsuke Chiba of Nanyang Technological University developed
(J. Am. Chem. Soc. 2009, 131, 12570.
DOI: 10.1021/ja905110c)
the Mn (III)-mediated fusion of a
cyclopropanol 18 with an alkenyl azide
19 to deliver the pyridine 20. Kazuaki Shimada of Iwate University found
(Tetrahedron Lett. 2009, 50, 6651.
DOI: 10.1016/j.tetlet.2009.09.060)
that an isotellurazole such as 21, easily prepared from the corresponding alkyne,
condensed with another alkyne 22, delivering the pyridine 23 with
high regiocontrol. Christopher J. Moody of the University of Nottingham devised
(Org. Lett. 2009, 11, 3686.
DOI: 10.1021/ol901502u)
a new route to the 1,2,4-triazine 24 from an α-diazoacetoacetate. He carried
24 on to the pyridine 26 by condensation with norbornadiene 25.
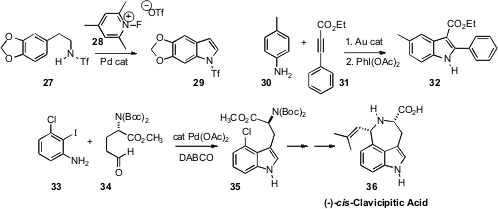
Extending his studies of selective ortho palladation, Jin-Quan Yu of Scripps/La Jolla observed
(J. Am. Chem. Soc. 2009, 131, 10806.
DOI: 10.1021/ja904709b)
that the triflamide 27 was converted to the
indole 29.
Troels Skrydstrup of Aarhus University described
(Org. Lett. 2009, 11, 4208.
DOI: 10.1021/ol901565p)
the regioselective Au-mediated addition of an aniline 30 to the alkyne 31
followed by oxidation, to give the indole 32. A key step in the total synthesis
(J. Org. Chem. 2009, 74, 6859.
DOI: 10.1021/jo9012755)
of (-)-cis-Clavicipitic Acid (36) by Yanxing Jia of Peking University was the
Pd-mediated combination of the iodo aniline 33 with the aldehyde 34 to give 35.