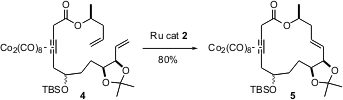We last reviewed organic synthesis applications of the Grubbs reaction on April 19, 2004. The (relatively) robust nature of the commercially-available catalyst and its commercial availability have spurred the expanding exploration of the scope of this reaction. This month, we are featuring some recent highlights. (Part One / Part Three). PMID:27641997 BuyTriazabicyclodecene
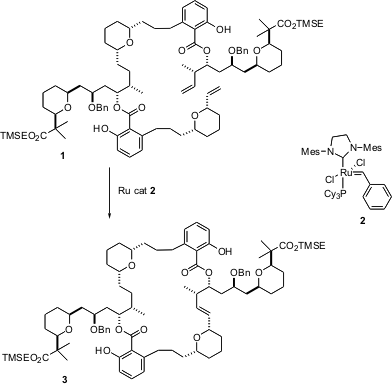
There has been a great deal of effort over the past several years directed toward the total synthesis of physiologically-activemacrolactones and macrolactams. 3,5-Dichloropyrido[3,4-b]pyrazine uses The availability and functional group tolerance of the Grubbs catalysts, especially the second generation Grubbs catalyst2, now make alkene metathesis an attractive procedure for closing the macrocyclic ring. Eun Lee of Seoul National University, in the course of a total synthesis of (+)-SCH 351444, a novel activator of LDL-R promoter, reported (J. Am. Chem. Soc. 2004, 126, 2680.DOI: 10.1021/ja0315309)the cyclization of 1 to 3.
Other alkenes in the substrate, and especially other alkynes, will react with the Ru catalyst. Samuel Danishefsky, of Columbia University and Sloan-Kettering Institute, recently completed (Org. Lett. 2004, 6, 413.DOI: 10.1021/ol036258m)the total synthesis of the antimalarial and antitumor agent aigialomycin D. For the Ru-mediated macrocyclization of 4 to 5, the alkyne in the molecule was protected as the dicobalt octacarbonyl adduct. After the cyclization, the alkyne was deprotected by brief exposure to ceric ammonium nitrate.
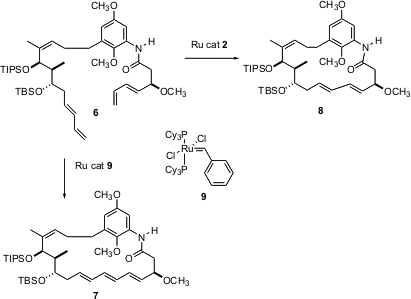
Selectivity can sometimes be achieved by changing the Ru catalyst. James Panek of Boston University, reported (Org. Lett. 2004, 6, 525.DOI: 10.1021/ol036284k)that the attempted conversion of 6 to the tetraene macrolactam core 7 of the cyclotrienins led instead to the unwanted8. Use of the less-reactive first generation Grubbs catalyst 9 gave clean cyclization to the desired7.