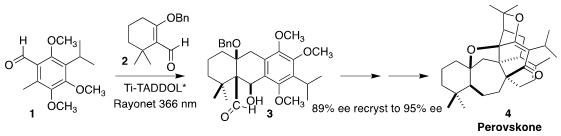
Perovskone (4), isolated from Perovskia abratanoides, contains a complex
heptacyclic framework and eight adjacent stereogenic centers. En route to 4
and other Salvia triterpenoids, Shuanhu Gao of East China Normal
University set the absolute configuration of the ring system by photolysis of
the Ti-TADDOL complex of the aldehyde 1 with the aldehyde 2
(J. BrettPhos Pd G3 custom synthesis Am. Chem. Soc. PMID:22943596 BuyMethyl aminolevulinate (hydrochloride) 2021, 143, 6370.
DOI: 10.1021/jacs.1c02674).
Majetich described an alternative strategy (not illustrated) for
establishing the absolute configuration of these natural products
(Tetrahedron 2011, 67, 10129.
DOI: 10.1016/j.tet.2011.09.072).
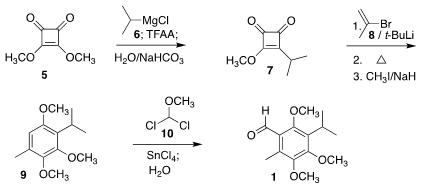
The aldehyde 1 was readily prepared following the Moore protocol from
commercial dimethyl squarate 5. Addition of isopropyl Grignard 6
followed by trifluoroacetic anhydride led to 7. Addition of the Li
reagent derived from 8 followed by thermal rearrangement and methylation
gave 9, that was formylated with 10 to give 1.
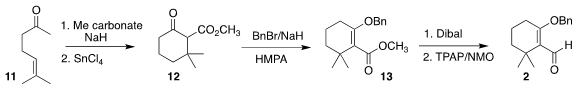
The preparation of the aldehyde 2 began with the inexpensive ketone
11. The derived β-keto ester was cyclized to
12, that was O-benzylated,
leading to the enol ether 13. Reduction followed by oxidation then completed the
synthesis of 2.
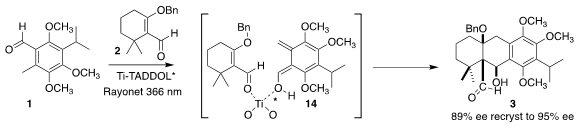
The photochemically-driven combination of 1 and 2 was envisioned as
proceeding via the photo enol 14. The chiral backbone of the Ti complex imparts
a twist to that intermediate, favoring one diastereomer over the other. The
crystalline product 3 was readily brought to near enantiopurity.
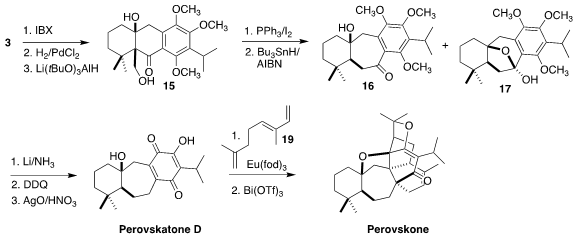
The aldehyde 3 was oxidized, then
debenzylated. The resulting keto aldehyde
was selectively reduced to the alcohol
15. Conversion to the iodide followed by
free radical reduction led, via Dowd-Beckwith rearrangement, to the
ring expanded ketone
16 along with its hemiketal 17. Dissolving metal reduction of
the mixure removed the benzylic oxygenation. Oxidation then led to the quinone
18, the natural product perovskatone D.
Following the Majetich precedent, intermolecular Diels-Alder reaction of the
quinone 18 with trans-ocimene (19) followed by acid-catalyzed cyclization gave the
heptacyclic natural product perovskone (4). This approach also enabled the
synthesis ofs several other of the Salvia triterpenoids.