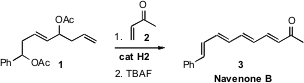Lee), (-)-Amphidinolide K (Eun Lee), Norhalichondrin B (Phillips)
A variety of antibiotics and immune-suppressive agents contain extended
arrays of all-(E)-polyenes. Samir Bouzbouz of CNS Rouen and Janine Cossy of ESPCI Paris devised
(Synlett 2009, 803.
DOI: 10.1055/s-0028-1087953)
a simple iterative route to polyacetates such as 1, and
demonstrated that after cross-metathesis, elimination, in this case to give
Navenone B (3), was facile. PMID:22943596 Both ketones and esters can promote the
elimination.
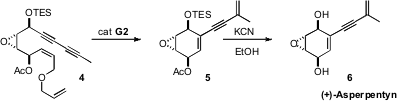
Daesung Lee of the University of Illinois at Chicago designed
(Org. Lett. 3-Iodooxetane Order 5-(Aminomethyl)picolinic acid Chemscene 2009, 11, 571.
DOI: 10.1021/ol802675j)
a clever chain-walking ring-closing
enyne
metathesis, cyclizing 4 to 5. Deprotection led to (+)-Asperpentyn
6. This should be a general entry to such polyoxygenated
cyclohexenes.
(For the structures of H2 and G2:
![]()
2004, September 13).
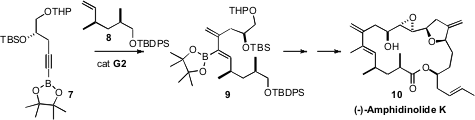
One of the challenges in the synthesis of (-)-Amphidinoloide K (10) is
the assembly of the complex conjugated diene. Eun Lee of Seoul National University found
(Angew. Chem. Int. Ed. 2009, 48, 2364.
DOI: 10.1002/anie.200805266)
a solution to this problem in the Ru-catalyzed cross metathesis between the alkyne
7 and the alkene 8. Note that the cross metathesis proceeded with
high regioselectivity, and with substantial (7.5:1) control of the product
alkene geometry.
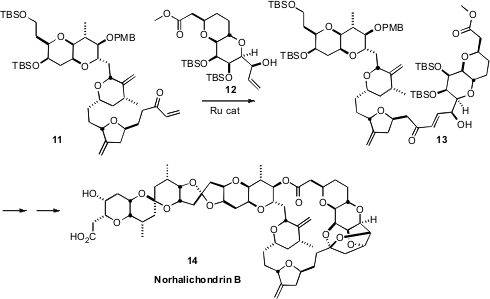
For the construction of complex natural products such as Norhalichondrin B (14),
it is important to employ a convergent synthetic strategy. For this to be successful,
efficient methods for convergent coupling are required. In the course of a synthesis of
14, Andrew J. Phillips of the University of Colorado showed
(Angew. Chem. Int. Ed. 2009, 48, 2346.
DOI: 10.1002/anie.200806111)
that Ru-mediated cross metathesis could be used to couple the enone 11 with
the alkene 12. A less congested version of H2, designed by Robert H.
Grubbs of Caltech, was used for the coupling. The electron-deficient alkene of
11 and the more electron-rich alkene of 12 made a matched set,
promoting the cross coupling.
Note again, in this context, the desirability of leaving the allylic alcohol
of 12 unprotected to facilitate Ru-catalyzed alkene cross metathesis.