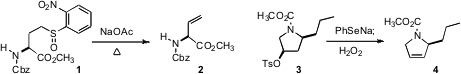Vinyl glycine 2 is a useful precursor to a variety of amino acids. PMID:23789847
Timothy E. Long of the University of Georgia found
(Tetrahedron Lett. 2009, 50, 5067.
DOI: 10.1016/j.tetlet.2009.06.082)
that the o-nitrophenyl sulfoxide 1 eliminated smoothly in refluxing
toluene. Alicia Boto and Rosendo Hernández of IPNA La Laguna observed
(Tetrahedron Lett. Bromo-PEG3-C2-acid custom synthesis 2009, 50, 3974.
DOI: 10.1016/j.tetlet.2009.04.082)
that a related selenoxide elimination proceeded to give the single regioisomer 4.
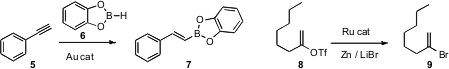
Avelino Corma of the Universidad Politécnica de Valencia developed
(Chem. Commun. 2009, 4947.
DOI: 10.1039/b901953g)
a gold catalyst for the selective hydroboration of alkynes over alkenes.
Eiji Shirakawa and Tamio Hayashi of Kyoto University devised
(Chem. Commun. 2009, 5088.
DOI: 10.1039/b907761h)
a Ru catalyst for the conversion of an alkenyl triflate such as 8
to the corresponding bromide. 42225-04-7 Chemscene
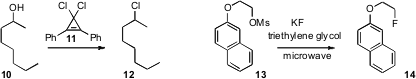
Tristan H. Lambert of Columbia University found
(J. Am. Chem. Soc. 2009, 131, 13930.
DOI: 10.1021/ja906520p)
that the dichloride 11 smoothly converted a
variety of alcohols into the corresponding
chlorides. Crown ethers have been
used to promote
SN2 reactivity by solubilizing the metal cation.
Sungyul Lee of Kyunghee University, Dae Yoon Chi of Sogang University, and
Choong Eui Song of Sungkyunkwan University demonstrated
(Angew. Chem. Int. Ed. 2009, 48, 7683.
DOI: 10.1002/anie.200903903)
that the inexpensive polyethylene glycols were also effective.
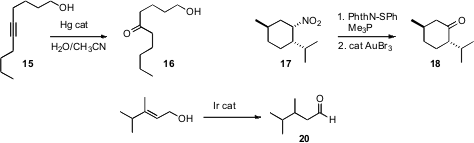
Mugio Nishizawa of Tokushima Bunri University devised
(Synlett 2009, 1175.
DOI: 10.1055/s-0028-1088153)
conditions for the rapid, regioselective hydration of hydroxy alkynes such
as 15. Jaume Vilarrasa of the Universitat de Barcelona developed
(Org. Lett. 2009, 11, 4414.
DOI: 10.1021/ol9017722)
a mild, alternative protocol for the
Nef reaction, converting a nitroalkane such as 17
into the corresponding ketone under neutral conditions. Clément Mazet of the University of Geneva optimized
(Tetrahedron Lett. 2009, 50, 4141.
DOI: 10.1016/j.tetlet.2009.04.130)
the Ir-catalyzed conversion of an allylic alcohol 19 into the saturated aldehyde.
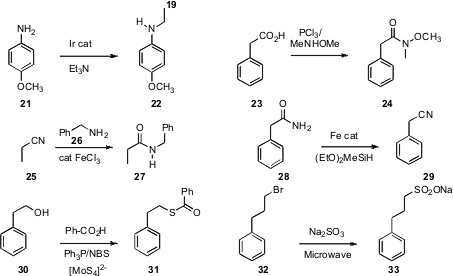
Jonathan M. J. Williams of the University of Bath established
(Angew. Chem. Int. Ed. 2009, 48, 7375.
DOI: 10.1002/anie.200904028)
that under Ir-catalyzed “borrowing hydrogen” conditions, alkyl amines could
donate alkyl groups to anilines such as 21. Danfeng Huang and Yulai
Hu of Northwest Normal University devised
(Org. Lett. 2009, 11, 4474.
DOI: 10.1021/ol901886u)
a simple protocol for conversion of an acid 23 to the Weinreb amide 24.
Professor Williams found
(Tetrahedron Lett. 2009, 50, 4262.
DOI: 10.1016/j.tetlet.2009.05.021)
that FeCl3 was an effective catalyst for the acylation of an amine
26 with a nitrile 25. Matthias Beller of the Universität Rostock optimized
(Chem. Commun. 2009, 4883.
DOI: 10.1039/b910145d)
the Fe-mediated conversion of a primary amide 28 to the nitrile 29.
Srinivasan Chandrasekaran of the Indian Institute of Science Bangalore developed
(Eur. J. Org. Chem. 2009, 6043.
DOI: 10.1002/ejoc.200900956)
a simple procedure for conversion of an alcohol 30 to the thioester.
Spyros P. Nikas and Alexandros Makriyannis of Northeastern University established
(Tetrahedron Lett. 2009, 50, 7028.
DOI: 10.1016/j.tetlet.2009.09.167)
microwave conditions for the conversion of a bromide 32 to the sulfonic acid salt 33.