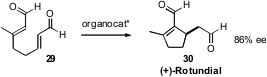(+)-Rotundial
Karl Anker Jørgensen of Aarhus University found
(Angew. Chem. Int. 1075198-30-9 Formula Ed. 2009, 48, 6650.
DOI: 10.1002/ange.200903253)
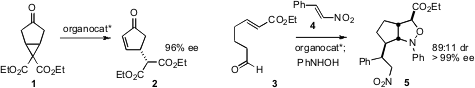
that an organocatalyst could mediate the fragmentation of the prochiral
cyclopropane 1 with high ee to the easily-epimerized product 2.
Guofu Zhong of Nanyang Technological University devised
(Angew. Chem. Int. Ed. 2009, 48, 6089.
DOI: 10.1002/ange.200901249)
a dipolar cycloaddition strategy for the organocatalyzed combination of 3
and 4 with PhNHOH to give the highly-substituted
cyclopentane 5.
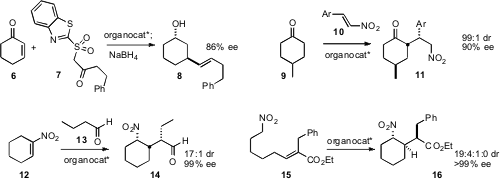
Professor Jørgensen also established
(Angew. Chem. 3-Oxoisoindoline-5-carbaldehyde In stock Int. Ed. 2009, 48, 7338.
DOI: 10.1002/anie.200903790)
that conjugate addition of 7 to the prochiral
cyclohexenone 6
proceeded with high ee. The initial adduct could be converted into the alkene
8, the alkyne, or the ketone. Wen-Jing Xiao of Central China Normal
University, following up on the work of Gong and Cheng, developed
(Tetrahedron 2009, 65, 9238.
DOI: 10.1016/j.tet.2009.09.005)
a simple organocatalyst for the desymmetrizing
Michael addition of 9
to 10 to give 11 with high de and ee. PMID:23557924
Control of sidechain chirality is an important aspect of
carbocyclic construction. Samuel H. Gellman of the University of Wisconsin demonstrated
(J. Am. Chem. Soc. 2009, 131, 16018.
DOI: 10.1021/ja907233q)
that the organocatalyzed addition of 13 to 12 proceeded with high
facial selectivity and excellent diastereocontrol. In a complementary approach,
Alexander J. A. Cobb of the University of Reading optimized
(J. Am. Chem. Soc. 2009, 131, 16016.
DOI: 10.1021/ja9070915)
an organocatalyst for the cyclization of 15 to 16,
again with high facial selectivity and excellent diastereocontrol.
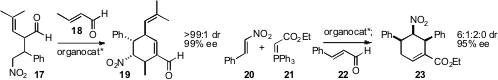
Ying-Chun Chen of the West China School of Pharmacy established
(Org. Lett. 2009, 11, 4660.
DOI: 10.1021/ol901939b)
conditions for the organocatalyzed combination of 17 with
18 to give 19. In a related approach, Bor-Cherng
Hong of the National Chung Cheng University showed
(Org. Lett. 2009, 11, 5246.
DOI: 10.1021/ol9021832)
that 20, 21 and 22 could be combined,
under organocatalysis, to give 23 in high ee with excellent
diastereocontrol. Both of these approaches, and several others that have been
published recently, were carried out with aryl substituents. It remains to be
seen whether alkyl substituents, that would be more useful in a target-directed
synthesis, would be compatible with these methods for ring construction.
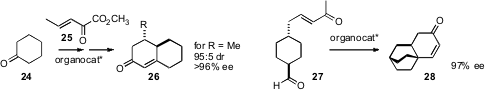
Synthetic strategies for the construction of polycarbocycles are also
important. Gilles Dujardin of the Université de Maine found
(Org. Lett. 2009, 11, 3060.
DOI: 10.1021/ol901065e)
that the activated Michael acceptor 25
condensed efficiently with the enamine derived from the combination of
cyclohexanone 24 with an organocatalyst. One initial adduct (R = [MeO]3Ph)
was carried on to the octalone 26. In this case, a full equivalent of the
organocatalyst was employed, but it could be recovered and reused.
Hisashi Yamamoto of the University of Chicago devised
(Chem. Commun. 2009, 5412.
DOI: 10.1039/B912325C)
an organocatalyst for the enantioselective intramolecular
Michael addition of the prochiral keto aldehyde 27. Intramolecular
aldol
condensation of the initial adduct completed the preparation of the tricyclic
enone 28.
The β-substituted aldehyde of 29 is reluctant to participate
in Michael addition. Mathias Christmann of the TU Dortmund took advantage
(Org. Lett. 2009, 11, 4116.
DOI: 10.1021/ol901614t)
of this in devising the organocatalyzed cyclization of 29
directly to the Vitex rotundifolia iridoid (+)-Rotundial (30).