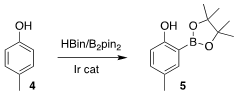Of the several hamigerans isolated from the marine sponge Hamigera
tarangaensis, only two contain nitrogen. Mingji Dai of Purdue University
devised a route to one of those, hamigeran M (3), using the
oxaxole ring
as a central organizing element
(J. Am. Chem. Soc. 2021, 143, 20084.
DOI: 10.1021/jacs.1c11060). 4-Bromo-2-chloro-6-fluorobenzaldehyde Chemscene
The benzene ring of 3 was prepared by Ir-catalyzed borylation of p-cresol 4. Formula of 1,3,6,8-Tetrakis[p-benzoic acid]pyrene
The borate 5 was stable to purification by silica gel chromatography. PMID:23935843
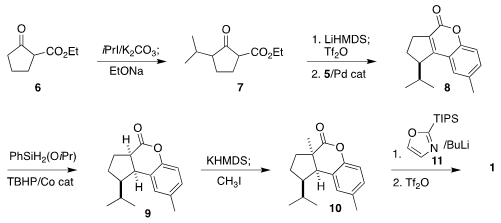
Alkylation of commercial 6 followed
by retro-Dieckmann/Dieckmann
reorganization delivered the β-keto ester 7. Coupling of the derived enol triflate
with 5 led to the lactone 8. After some experimentation, diastereoselective
reduction to 9 was accomplished by Co-catalyzed hydrogen-atom-transfer (HAT). Alkylation
to 10 followed by combination with the metalated oxazole 11 completed the assembly
of the triflate 1.
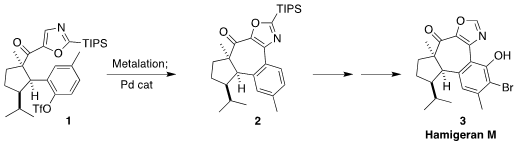
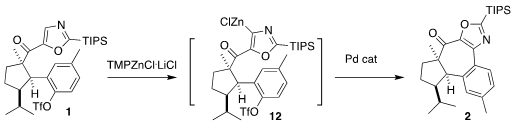
Further metalation of
the oxazole 1 led to the organozinc 12, that was cyclized via Pd-catalyzed
Negishi coupling to the
cycloheptanone 2.
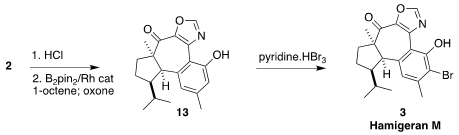
To complete the synthesis, the TIPS group
was removed. Rh-catalyzed ortho
borylation followed by oxidation then delivered
the phenol 13. Bromination
substantially favored the ortho position, to give hamigeran M (3).
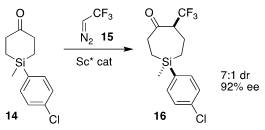
Enantioenriched organosilanes have great potential for pharmaceutical
development. Jianbo Wang of Peking University demonstrated that
ring expansion
of the prochiral ketone 14 with trifluoromethyl diazomethane 15 led to cyclic
silane 16 in high de and ee
(Angew. Chem. Int. Ed. 2022, 61, e202115098.
DOI: 10.1002/anie.202115098).
Frances Arnold of Caltech reviewed biocatalytic transformations of silicon
(ACS Central Sci. 2021, 7, 944.
DOI: 10.1021/acscentsci.1c00182).