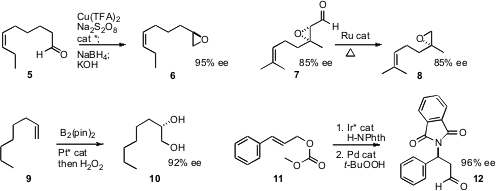
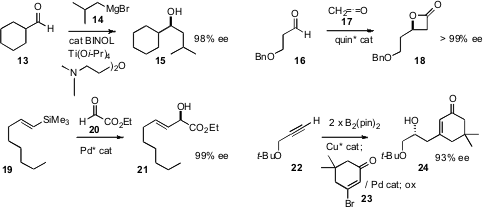
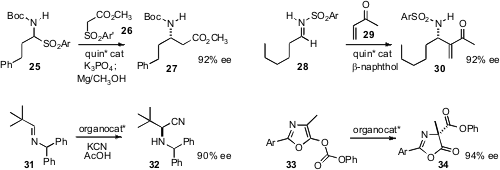
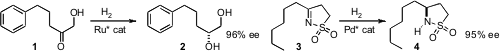Renat Kadyrov of Evonik Degussa and Magnus Rueping of RWTH Aachen developed
(Angew. Chem. Int. Ed. 2009, 49, 7556.
DOI: 10.1002/anie.200902835)
an effective catalyst for the enantioselective hydrogenation of an α-hydroxy ketone 1 to
the 1,2-diol 2. Yong-Gui Zhou of the Dalian Institute of Chemical Physics showed
(J. Org. Chem. 2009, 74, 5633.
DOI: 10.1021/jo900790k)
that a sultam such as 3 could be reduced with high ee to the
sulfonamide 4. They also used this same approach to prepare both α-aryl and α,α-diaryl
amines. 2089292-48-6 Chemscene
David W. C. MacMillan of Princeton University described
(Angew. Chem. Int. Ed. Formula of (3-(4-Hydroxyphenyl)acryloyl)glycine 2009, 49, 5121.
DOI: 10.1002/anie.200901855)
the optimized enantioselective α-chlorination of an aldehyde 5 and the direct
processing of the product to the epoxide 6. Erick M. PMID:23537004 Carreira of ETH Zürich reported
(Synlett 2009, 2076.
DOI: 10.1055/s-0029-1217562)
an alternative route to high ee epoxides by decarbonylation of an epoxy aldehyde 7.
James P. Morken of Boston College established
(J. Am. Chem. Soc. 2009, 131, 13210.
DOI: 10.1021/ja9047762)
a procedure for the enantioselective bis borylation of a terminal alkene 9, leading after oxidation
to the 1,2-diol 10. Ben L. Feringa of the University of Groningen took advantage
(J. Am. Chem. Soc. 2009, 131, 9473.
DOI: 10.1021/ja902591g)
of their alternative Wacker conditions to convert a primary allylic carbonate 11 to the
protected β-amino aldehyde12.
Chao-Shan Da of Lanzhou University devised
(Org. Lett. 2009, 11, 5578.
DOI: 10.1021/ol9020942)
additives that allow the direct enantioselective
addition of a Grignard reagent
14 to an aldehyde. The enantioselective addition of substituted ketenes to
aldehydes has long been established. Yun-Ming Lin of the University of Toledo developed
(Synlett 2009, 1675.
DOI: 10.1055/s-0029-1217332)
a catalyst system for the enantioselective addition of ketene 17 itself.
An alkenyl silane 19 can readily be prepared from the corresponding terminal alkene
(J. Org. Chem. 2010, 75, 1701.
DOI: 10.1021/jo902678p).
Koichi Mikami of the Tokyo Institute of Technology showed
(J. Am. Chem. Soc. 2009, 131, 13922.
DOI: 10.1021/ja906164p).
that such alkenyl silanes add to ethyl glyoxylate 20 with high ee.
Amir H. Hoveyda of Boston College devised
(J. Am. Chem. Soc. 2009, 131, 18234.
DOI: 10.1021/ja9089928)
a procedure for the enantioselective conversion of a terminal alkyne 22
to the 1,2-bis boryl alkane, that he took on directly to the coupled product 24.
Note that these are the same sort of 1,2-bis boryl alkanes as those described by
Professor Morken in the conversion of 9 to 10.
Luca Bernardi and Alfredo Ricci of the University of Bologna optimized
(Angew. Chem. Int. Ed. 2009, 49, 5694.
DOI: 10.1002/anie.200900701)
a quinidine-based catalyst for the enantioselective addition of the commercial 26 to the aldehyde derivative
25 to give the β-amido ester 27. Géraldine Masson and Jieping Zhu of Gif-sur-Yvette reported
(Org. Lett. 2009, 11, 4648.
DOI: 10.1021/ol901920s)
a related catalyst for the enantioselective aza-Morita-Baylis-Hillman addition of 29 to the imine 28.
Eric N. Jacobsen of Harvard University developed
(Nature 2009, 461, 968.
DOI: 10.1038/nature08484)
a practical procedure for the
Strecker cyanation of the pivaldehyde imine 31.
Andrew D. Smith of the University of St. Andrews designed
(Angew. Chem. Int. Ed. 2009, 49, 8914.
DOI: 10.1002/anie.200904333)
an organocatalyst for the enantioselective rearrangement of 33 to
34.