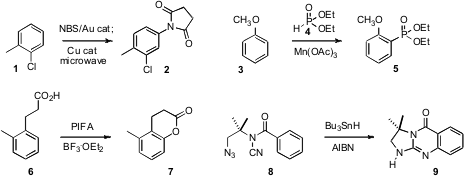
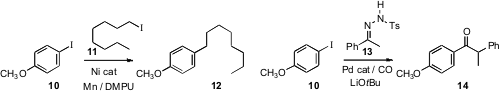
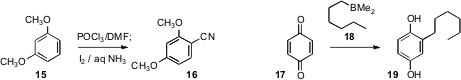
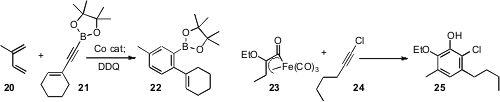
Jianbo Wang of Peking University described
(Angew. Chem. Int. Ed. 2010, 49, 2028.
DOI: 10.1002/anie.200906699)
the Au-promoted bromination of a benzene derivative such as 1 with
N-bromosuccinimide.
In a one-pot procedure, addition of a Cu catalyst followed by microwave heating delivered
the aminated product 2. Jian-Ping Zou of Suzhou University and Wei Zhang of the
University of Massachusetts, Boston, observed
(Tetrahedron Lett. 2010, 51, 2639.
DOI: 10.1016/j.tetlet.2010.03.029)
that the phosphonylation of an arene 3 proceeded with substantial ortho selectivity.
Yonghong Gu of the University of Science and Technology, Hefei showed
(Tetrahedron Lett. 2010, 51, 192.
DOI: 10.1016/j.tetlet.2009.10.112)
that an arylpropanoic acid 6 could be ortho hydroxylated with
PIFA, to give 7. 2621939-48-6 Formula Louis Fensterbank, Max Malacria and Emmanuel Lacôte
of UMPC Paris found
(Angew. Chem. 1951466-68-4 Order Int. Ed. PMID:23626759 2010, 49, 2178.
DOI: 10.1002/anie.200907237)
that a benzoic acid could be ortho aminated by way of the cyano amide 8.
Daniel J. Weix of the University of Rochester developed
(J. Am. Chem. Soc. 2010, 132, 920.
DOI: 10.1021/ja9093956)
a protocol for coupling an aryl iodide 10 with an alkyl iodide 11
to give 12. Professor Wang devised
(Angew. Chem. Int. Ed. 2010, 49, 1139.
DOI: 10.1002/anie.200906349)
a mechanistically-intriguing alkyl carbonylation of an iodobenzene 10.
This is presumably proceeding by way of the intermediate diazo alkane.
Usually, benzonitriles are prepared by cyanation of the halo aromatic. Hideo
Togo of Chiba University established
(Synlett 2010, 1067.
DOI: 10.1055/s-0029-1219575)
a protocol for the direct electrophilic
cyanation of an electron-rich aromatic
15. Thomas E. Cole of San Diego State University observed
(Tetrahedron Lett. 2010, 51, 3033.
DOI: 10.1016/j.tetlet.2010.03.096)
that an alkyl dimethyl borane, readily prepared by
hydroboration of the alkene with BCl3 and Et3SiH, reacted with benzoquinone (17)
to give 18. Presumably this transformation could also be applied to substituted benzoquinones.
When a highly substituted benzene derivative is needed, it is sometimes more
economical to construct the aromatic ring. Joseph P. A. Harrity of the
University of Sheffield and Gerhard Hilt of Philipps-Universität Marburg showed
(J. Org. Chem. 2010, 75, 3893.
DOI: 10.1021/jo1004907)
that the Co-catalyzed Diels-Alder cyloaddition of alkynyl borinate 21 with
a diene 20 proceeded with high regiocontrol, to give, after oxidation, the
aryl borinate 22. Wayne F. K. Schnatter of Long Island University found
(Tetrahedron Lett. 2010, 51, 921.
DOI: 10.1016/j.tetlet.2009.12.029)
that the addition of the readily-prepared Fe complex 23 to 24 also
proceeded with high regioselectivity, to give the pentasubstituted benzene 25.
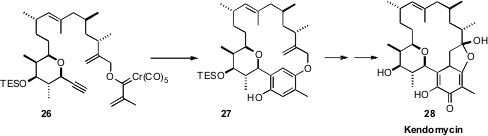
Yoko Saikawa and Masaya Nakata of Keio University took advantage
(Org. Lett. 2010, 12, 1700.
DOI: 10.1021/ol100229f)
of arene construction in their synthesis of Kendomycin (28). Intramolecular Dötz
cyclization of the Fischer carbene 26 gave 27, poised for the
Claisen rearrangement
that would establish the last C-C bond on the aromatic ring of the natural product.
We salute Professors Heck, Negishi and Suzuki for the 2010 Nobel Prize in
Chemistry, for the development of Pd-catalyzed bond construction.