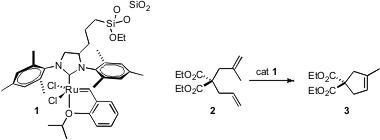
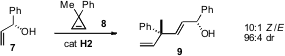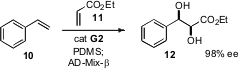There are two major impediments to the scaling up of
alkene metathesis, reducing the amount of the expensive Ru catalyst required, and minimizing
residual Ru in the product. Robert H. PMID:24238102 Grubbs of Caltech developed
(Org. Lett. 2009, 11, 1261.
DOI: 10.1021/ol9000153)
a family of silica-supported Ru complexes,
exemplified by 1. At 0.75 mol % of 1, the rate of cyclization of
2 to 3 was maintained over eight cycles. The solution of product
3 showed < 5 ppb Ru. Hassan S. Bazzi and David E. Bergbreiter of the
Texas A& M campuses in Qatar and College Station also reported
(Org. Lett. 2009, 11, 665.
DOI: 10.1021/ol802728t)
a durable polymer-bound Ru metathesis catalyst that
maintained its activity over many cycles. Ir[dF(F)ppy]2(dtbbpy)PF6 In stock
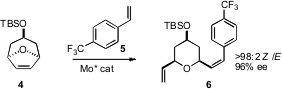
Most metathesis catalysts are strongly E selective. Amir H. Hoveyda of
Boston College designed
(J. Am. Chem. (3-Bromo-1-propyn-1-yl)cyclopropane site Soc. 2009, 131, 3844.
DOI: 10.1021/ja900097n)
a chiral Mo catalyst that was both highly enantioselective and strongly Z
selective, converting the prochiral 4 into the alkene 6.
Professor Hoveyda also took advantage
(J. Am. Chem. Soc. 2009, 131, 8378.
DOI: 10.1021/ja9030903)
of the known propensity of Ru metathesis catalysts for H
bonding, showing that metathesis of the prochiral cyclopropene 7
proceeded with remarkable diastereocontrol. This appears to be a generally
useful protocol for assembling enantiomerically-pure alkylated quaternary
stereogenic centers.
It is also possible to encapsulate the Ru catalyst. Ned B. Bowden of the University
of Iowa pioneered the use of PDMS thimbles for this purpose. He has now shown
(Org. Lett. 2009, 11, 33.
DOI: 10.1021/ol8022215)
that by subsequently adding AD-mix,
cross metathesis can be followed
directly by enantioselective dihydroxylation.
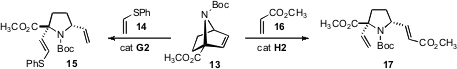
Ring-opening cross metathesis of an unsymmetrical alkene such as 13
could give two different products. Alberto Avenoza and Jesús H. Busto of the
Universidad de La Rioja established
(J. Org. Chem. 2009, 74, 1736.
DOI: 10.1021/jo802399k)
that by tuning the electronic nature of the participating alkene, either
product can be obtained with high selectivity.
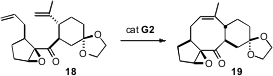
Metathesis can be used to close larger rings. Conformational effects are
important. Motoo Tori of Tokushima Bunri University observed
(Tetrahedron Lett. 2009, 50, 2225.
DOI: 10.1016/j.tetlet.2009.02.163)
that while 18 cyclized efficiently, the other three precursors that were
diastereomeric on the cyclopentane ring did not undergo ring-closing metathesis.
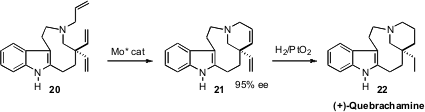
To accomplish the enantioselective construction of the Aspidosperma alkaloid
(+)-Quebrachamine 22, Professor Hoveyda envisioned
(J. Am. Chem. Soc. 2009, 131, 943.
DOI: 10.1021/ja8084934)
the selective ring-closing metathesis of 20
to 21. The stereogenic-at-Mo complex he and his co-workers developed was
also effective in other applications, including the ring-opening cross
metathesis of 4.