Euonyminol (3) was isolated from the thunder duke vine, Tripterygium wilfordii,
used in traditional Chinese medicine. In the course of a synthesis of 3, Seth B. Herzon of Yale University established the key cyclic quaternary center by the
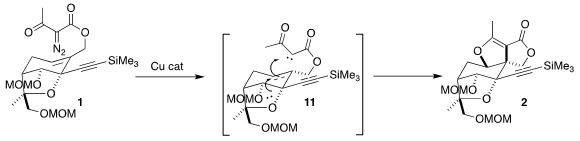
Cu-catalyzed cyclization of the α-diazo acetoacetate
1 to the enol ether 2
(J. Am. Chem. Soc. 2021, 143, 699,
DOI: 10.1021/jacs.0c12998;
J. Org. SC209 intermediate-1 Formula Chem. PMID:23746961 2021, 86, 17011,
DOI: 10.1021/acs.joc.1c02167).
The preparation of the ester 1 began with commerical carvone 4. Following the
method of Floreancig, α-oxygenation led to the undesired diastereomer
5, but
that could be corrected by deprotonation/protonation.
Epoxidation then gave 6. Formula of Nα,Nα-Bis(carboxymethyl)-L-lysine
Addition of 7 followed by cyclization and protection led to 8, that was oxidized
to 9. Acylation with diketene 10 followed by
diazo transfer completed the
assembly of 1.
The Cu-catalyzed cyclization of 1 proceeded with substantial diastereocontrol to give the crystalline vinylogous carbonate
2. The authors rationalized this by postulating significant charge separation in the transition state
11. Stabilization of the incipient carbocation by the nonbonding electrons on the adjacent ether then blocked bond formation from that face, leading to the observed outcome.
Ozonolysis of
2 followed by
Baeyer-Villiger oxidation,
hydrolysis and
esterification gave the
monoacetate
12. Desilylation followed by oxidation and
the addition of vinyl magnesium bromide and acetonide formation then led to the
alkyne 13, that cyclized under free radical conditions to the
stannane 14, completing the carbon skeleton of
3. The later oxidative phase of the synthesis
depended on the 1,3-diol being protected by a bridging bis-silyloxy group. With
that group in place, however, the radical cyclization led to the alternative
cyclopentane. Only the
acetonide 13 cyclized to the desired
cyclohexane.
Destannylation and deprotection of 14 gave the intermediate diol, that was
carried on to the requisite cyclic siloxane 15. Oxidation to the enone followed
by methyl lithium addition and epoxidation then gave 16, with the stage set for
the completion of the synthesis of euonyminol (3).