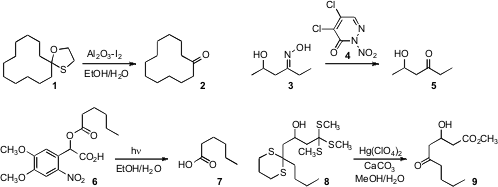
Bekington Myrboh of North-Eastern Hill University reported
(Tetrahedron Lett. 2010, 51, 2862.
DOI: 10.1016/j.tetlet.2010.03.084)
a convenient procedure for the oxidative removal of a 1,3-oxathiolane 1 or
a 1,3-dithiolane. Sang-Gyeong Lee and Yong-Jin Yoon of Gyeongsang National University developed
(J. Org. Chem. 2010, 75, 484.
DOI: 10.1021/jo902356e)
the pyridazin-3(2H)-one 4 for the
microwave-mediated deprotection of an oxime 3. Dario M. Bassani of
Université Bordeaux 1 and John S. Snaith of the University of Birmingham devised
(J. Price of 3-Amino-5-(tert-butyl)phenol Org. Chem. 2010, 75, 4648.
DOI: 10.1021/jo100783v)
a procedure for the facile preparation of esters such as 6. PMID:32472497 Brief photolysis
(350 nm) returned the parent carboxylic acid 7.
Craig M. Williams of the University of Queensland prepared
(Tetrahedron Lett. 2010, 51, 1158.
DOI: 10.1016/j.tetlet.2009.12.058)
the trithioorthoester 8 by iterative opening of epichlorohydrin.
He found that the keto ester 9 could be efficiently released by
Hg-mediated hydrolysis. Price of 1-(1H-indol-3-yl)-2-methylpropan-2-amine
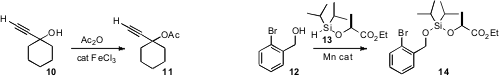
Masatoshi Mihara of the Osaka Municipal Technical Research Institute established
(Synlett 2010, 253.
DOI: 10.1055/s-0029-1219163)
that even very congested alcohols such as 10 could be
acetylated by
acetic anhydride containing a trace of FeCl3. Colleen
N. Scott, now at Southern Illinois University, developed
(J. Org. Chem. 2010, 75, 253.
DOI: 10.1021/jo9022765)
a convenient procedure for the preparation of the hydridosilane 13, that on Mn
catalysis added the alcohol 12 to make the unsymmetrical bisalkoxysilane
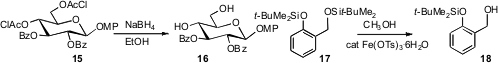
14. Sabine Berteina-Raboin of the Université d’Orléans found
(Tetrahedron Lett. 2010, 51, 2115.
DOI: 10.1016/j.tetlet.2010.02.057)
that NaBH4 in EtOH cleanly removed the chloroacetates from 15.
Both other esters and silyl ethers were stable under these conditions. Ram S. Mohan
of Illinois Wesleyan University established
(Tetrahedron Lett. 2010, 51, 1056.
DOI: 10.1016/j.tetlet.2009.12.076)
that Fe(III) tosylate in methanol selectively removed the
alkyl silyl ether from 17
without affecting the aryl silyl ether.
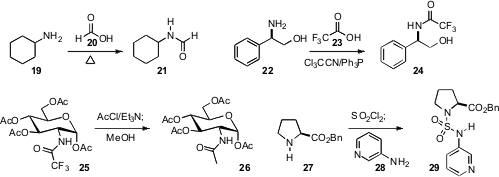
Alakananda Hajra and Adinath Majee of Visva-Bharati University effected
(Tetrahedron Lett. 2010, 51, 2896.
DOI: 10.1016/j.tetlet.2010.03.097)
formylation of an amine 19 by heating with commercial 85% formic acid as
the solvent in a sealed tube at 80°C. Although both primary and secondary amines
could be efficiently formylated, the primary amines were much more reactive.
Doo Ok Jang of Yonsei University found
(Tetrahedron Lett. 2010, 51, 683.
DOI: 10.1016/j.tetlet.2009.11.105)
that the conveniently-handled CF3CCO2H
(the acid chloride is a gas) could be activated in situ, to selectively convert 22 into
24. The importance of
trifluoroacetamides was underscored by the report
(Angew. Chem. Int. Ed. 2010, 49, 1850.
DOI: 10.1002/anie.200906055)
from Paola Rota of the University of Milan that 25 could be acylated with an
acid chloride. The trifluoroacetyl was then removed by mild hydrolysis,
completing the protecting group exchange without jeopardizing the esters.
Chuangxing Guo of Pfizer/San Diego observed
(Tetrahedron Lett. 2010, 51, 2909.
DOI: 10.1016/j.tetlet.2010.03.106)
that in situ coupling of the sulfamyl chloride derived from
27 with 28 in the presence of pyridine as the base gave low yields of
the coupled product, accompanied by a substantial amount of the undesired
primary mixed sulfamide. Using 3,5-lutidine as the base gave clean conversion to
29.