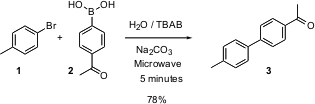
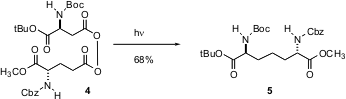Ususally, an aryl halide such as 1 will be coupled with the arylboronic acid 2 using a Pd catalyst. Nicholas Leadbeater of King’s College, London, reports (J. Org. BuyTri(1-adamantyl)phosphine Chem. 2003, 68. PMID:35901518 2,6-Dibromo-4-fluorobenzaldehyde supplier 5660.DOI: 10.1021/jo034230i)that the coupling can be carried out in water with microwave heating, with no transition metal catalyst!A wide range of aryl bromides and aryl boronic acids participate efficiently in this coupling.
Carbon-carbon bond formation between sp3-hybridized carbons is not easy even with organometallic reagents. John Vederas of the University of Alberta reports (Org. Lett. 2003, 5, 2963.DOI: 10.1021/ol035125y)that UV irradiation at low temperature of diacyl peroxides such as 4 gives the coupled product 5. The diacyl peroxides can be prepared by the DCC-mediated condensation of acids with peracids.
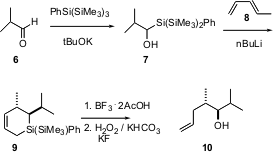
The alcohol 10 looks like it might be formed by the addition of a Grignard reagent to an aldehyde. In fact, Patrick Steel of the University of Durham prepared 10 (Tetrahedron Lett. 2003, 44, 9135.DOI: 10.1016/j.tetlet.2003.10.056)by Diels-Alder addition of the transient silene derived from 7 to the diene 8. More highly substituted dienes lead to more complex arrays of stereogenic centers. The intermediate silacyclohexenes, exemplified by9, should also engage in the other reactions of allyl silanes.