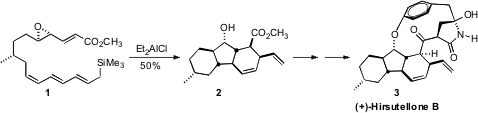
(+)-Hirsutellone B (3), isolated from the insect pathogenic fungus Hirsutella
nivea BCC 2594, shows good activity (MIC = 0.78 μg/mL) against Mycobacterium
tuberculosis. Approaching the synthesis of 3, K. Val-cit-PAB-OH Chemscene C. Nicolaou of Scripps/La Jolla
envisioned and reduced to practice
(Angew. Chem. Int. Ed. PMID:24238102 2009, 49, 6870.
DOI: 10.1002/anie.200903382)
a spectacular tandem intramolecular epoxide opening – internal
Diels-Alder
cyclization (1 → 2) that established all three of the
carbocyclic rings of
3 with the proper relative and absolute configuration. 2-Bromo-5-hydrazinylpyridine web
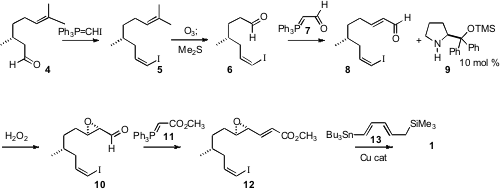
The construction of 1 began with commercial (R)-(+)-citronellal
(4). Wittig
homologation established the (Z)-iodide 5. Selective
ozonolysis followed by
condensation with the phosphorane 7 set the stage for Jørgensen-Córdova epoxidation
(Tetrahedron Lett. 2006, 47, 99.
DOI: 10.1016/j.tetlet.2005.10.128)
with H2O2 and a catalytic amount of the Hayashi
catalyst 9. Condensation of 10 with the phosphorane 11 followed by Cu-catalyzed
coupling of 12 with the organostannane 13 completed the assembly of
1.
This approach underscores the strategic advantages the Jørgensen-Córdova
epoxidation has over the
Sharpless protocol. It is not necessary to reduce the
aldehyde to the allyic alcohol, then reoxidize. Further, the Jørgensen-Córdova
epoxidation, using catalytic 9, is operationally easier than the Sharpless
procedure, that often uses stoichiometric amounts of tartrate ester.
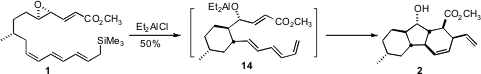
The cyclization of 1 proceeded by way of 14, with the newly formed
stereogenic center having the diene equatorial on the cyclohexane. Endo
cycloaddition catalyzed by the Lewis acid in the solution then gave 2. The
facility with which the cyclization of 14 set both the substituents and the
stereogenic centers of 2 raises the possibility that the biosynthesis might also
follow such an internal [4 + 2] cycloaddition.
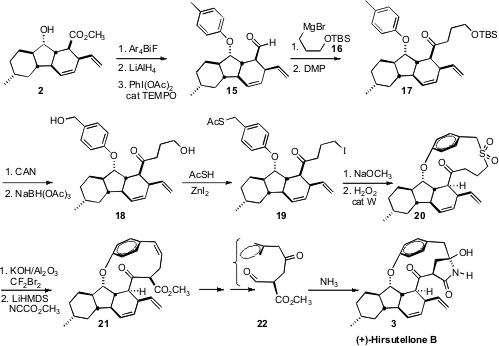
To complete the synthesis of 3, it was necessary to construct the strained
paracyclophane. The authors took advantage of the facile cyclization of the
thiolate liberated from 19, then installed the ring-contracted alkene with a
Ramberg-Bäcklund rearrangement. They completed the synthesis of (+)-Hirsutellone
B (3) by exposing the ketone 22 to NH3 in CH3OH/H2O.