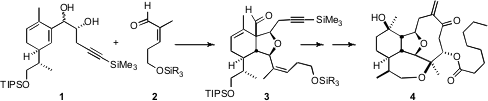
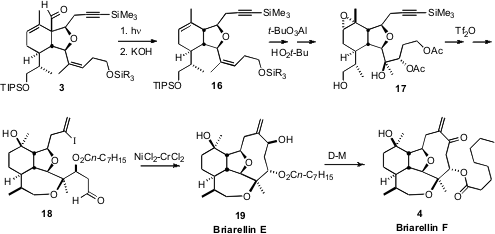
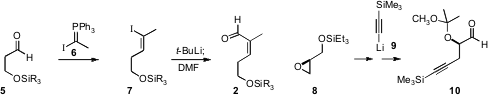Briarellin F (4) is an elegant representative of the complex
polycyclic ethers produced by soft corals such as Briareum abestinum.
Larry E. Overman of the University of California, Irvine developed (J. Org.
Chem. PMID:23558135 887144-97-0 Formula 2009, 74, 5458.
DOI: 10.1021/jo9010156)
a triply-convergent approach to 4,
the central feature of which was the
Prins-pinacol combination of 1 with
2 to give 3. 3-Chloro-5-nitro-1H-pyrazole Chemical name
The aldehyde 2 was assembled by
Wittig homologation of the aldehyde 5 with
the phosphorane 6, followed by metalation and formylation. The aldehyde
10 was prepared by opening the enantiomerically-pure epoxide 8 with the acetylide
9.
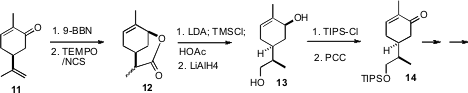
Hydroboration of carvone 11 could not be effected with sufficient
diastereocontrol. As an alternative, the mixture of diols was oxidized to the
lactone 12. Kinetic quench of the derived silyl ketene acetal followed by
reduction led to the diastereomerically-pure crystalline diol 13. This key
intermediate will have many other applications in target-directed synthesis.
The ketone 14 was converted to the alkenyl iodide 15 by stannylation of the
enol triflate, followed by exposure of the stannane to
N-iodosuccinimide.
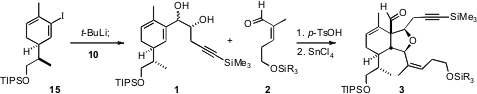
Addition of the alkenyl iodide 15 to the aldehyde 10 gave the diol
1 as an inconsequential 3:1 mixture of diastereomers. This mixture was combined with the
aldehyde 2 to give, via Lewis acid-mediated rearrangement of the
initially-prepared acetal, the aldehyde 3.
The aldehyde 3 was readily decarbonylated by irradiation in dioxane.
Face-selective Al-mediated epoxidation of the derived homoallylic alcohol
proceeded with 10:1 selectivity, and subsequent
MCPBA
epoxidation of the
cyclohexene was also secured with 10:1 facial control. This set the stage for
the triflic anhydride-mediated closure of the six-membered ring ether. The
Nozaki-Hiyama-Kishi cyclization of 18 proceeded with remarkable selectivity,
delivering Briarellin E (19) as a single diastereomer.
Dess-Martin oxidation converted 19 into Briarellin F (4).