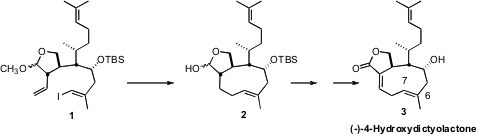
(-)-4-Hydroxydictyolactone (3), representative of the cyclononene
xenicanes isolated from the Dictyotacae algae, readily isomerizes
thermally to the more stable (Z)-6,7-isomer. Attempts to directly form
this strained ring system appeared to be fraught with difficulty. David R.
Williams of Indiana University envisoned
(J. Am. Chem. PMID:24856309 Price of H-Lys(Fmoc)-OH Soc. 2009, 131, 9038.
DOI: 10.1021/ja902677t)
that use of Suzuki coupling might ameliorate some of the
strain, since at the point of commitment to bond formation, the Pd center would
be included in the forming ring. This analysis led specifically to the trans
ether 1, as cyclization of the trans ether appeared likely to be more
facile than would cyclization of the alternative cis diastereomer. 1,18-Dibromooctadecane Price
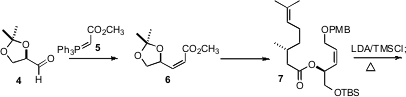
The first challenge was the assembly of the array the four contiguous
alkylated stereogenic centers of 1. To this end, the Z secondary
ester 7 was prepared from the acetonide 4, available from mannitol,
and (R)-(+)-citronellic acid, prepared by oxidation of the commercial
aldehyde. Addition of 7 to LDA led to decomposition, but inverse addition
of LDA to a mixture of the ester, TMSCl and Et3N smoothly delivered
the ketene silyl acetal. On warming,
Ireland-Claisen rearrangement of the ketene
silyl acetal led to the acid 8 with remarkable diastereocontrol.
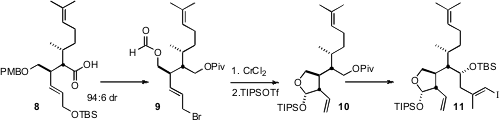
The last alkylated stereogenic center of 1 was installed by reductive
cyclization of the formate ester 9. Again, the cyclization proceeded with
remarkable diastererocontrol. While the intramolecular reaction of in situ
prepared allyl metals is well precedented, the addition to a formate ester had
not previously been reported.
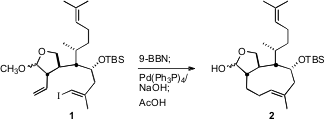
Although 11 appears to be ready for the long-awaited Suzuki coupling,
in fact the TIPS protecting group substantially slowed
hydroboration. The free
alcohol/methyl acetal was the best substrate for hydroboration, but the free
alcohol entered into other side reactions. After extensive experimentation, a
happy medium was found with the methyl acetal/TBS ether 1.
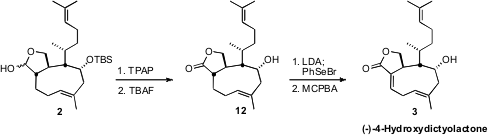
Selenylation of the lactone 12 followed by oxidative elimination of
the selenide delivered the expected Z alkene. Removal of the silyl
protecting group had to precede introduction of the second alkene, as the
product 3 deteriorated rapidly on exposure to the alkaline conditions of
TBAF cleavage.
Strained medium rings such as the (E,Z)-cyclononadiene assembled in
this study have been particularly challenging to prepare. It will be interesting
to see how generally useful the
Suzuki coupling turns out to be for the
construction of such rings.